Abstract
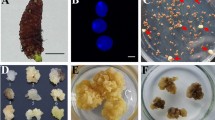
Gynogenetic haploid and double haploid (DH) lines produced earlier from six Monogerm and multigerm sugarbeet genotypes at Minsk, Belarus, were used in the present study with the objectives to study genetic variability of induced haploids and doubled haploid in sugarbeet populations, and to demonstrate genetic diversity of gynogenetic germplasm with help of molecular markers. Afterin vitro period of induction and micropropagation, plants of gynogenetic origin were characterized by low values of root yield and sugar content, due to mixoploid tissues. Prebreeding field trials of 40 genotypes (lines, their hybrids and controls) showed wide variability in double haploids for quantitative traits, with higher values for root weight, top weight and sugar yield/root in DH lines compared to haploids and their controls. Genetic diversity and variability among DH lines of different origin alongwith checks was studied through AFLP techniques. The phylogentic tree for 19 selected genotypes was constructed which gave interesting information and six lines were selected as base material for broadening of genetic base to produce experimental hybrids.
Similar content being viewed by others
References
Achard, F.C. (1979). Ausfuhrliche Beshreibung der Methode, nach welcher beider Kultur der Runkelsube verfahsen werfahren werden mulfb. C.S. Spencer, Berlin) pp 63.
Cooke, D.A. and Scott, R.K. (1993). Introduction. The Sugarbeet Crop pp xiv-ix Chapman & Hall, UK.
Bosemark, N.O. (1993). Genetics & Breeding pp 67–119. In the Sugarbeet Crop (Eds. D.A. Cook and R.K. Scott), Chapman & Hall.
Bossotrot, D. and Hosemans, D. (1985). Gynogenesis inBeta vulgaris, L. fromin vitro culture of unpollinated ovules to the production of doubled haploid plants in soil. Plant Cell Reptr.,4: 300–303.
Fronoghi-wehr and Zeller, A. (1990).In vitro micropore reaction of different german wheat cultivars. Theor. Appl. Genet,79: 77–80.
Finnie, S.J, Powell, W. and Dyer, A.F. (1989). The effect of carbohydrate composition and concentration on anther culture response in barley (Hordeum vulgaris, L.) Plant Breeding,103: 110–118.
Goska, M. and Jassem, B. (1988). Histological observations of Sugarbeet ovulesIn-vitro Cultures. Bull. Pol. Acad. Sci. Biol. Sci.,36: 167–175.
Hansen, A.L., Plaver, C., Pedersen, H.C., Keimer, H.C. and Anderson, S.B. (1994). Efficientin-vitro cromosome doubling duringBeta vulgaris, L. Chromosome doubling duringBeta vulgaris, L. ovule culture. Plant Breeding,112: 89–95.
Hansen, A.L., Gertz, A., Joersbo, M. and Anderson, S.B. (1995). Short duration colchiine treatment for in-vitro chromosome doubling during ovule culture ofBeta vulgaris, L.Plant Breeding,114: 515–519.
Hussey, G. and Hepher, A. (1978). Clonal propagation of sugar beet plants and the formation of polyploids by tissue culture. Annals of Bot.,42: 477–479.
Lindsey, K. and Jones, M.G.K. (eds) (1989). Plant Biotechnology in Agriculture. Open University Press, Milton Keynes, UK.
Mathias, R. and Robbelen, G. (1991). Effective diploidization of microspore-derived haploids of rape (Brassica napus, L.) byin-vitro cochincine treatment.Plant Breeding,106: 82–84.
Marggraf, A.S. (1779). Experiences chymiques faites dans le dessein de tirer un vere table sucre de diverses plantes, quis croissent dans nos coulrees. In Histoire de 1 Academie Royale des Sciences et Bells Lellres, Berlin: pp 79–90.
McGrath, J.M., de Los Reyes B. and Saunders J.W. (2000). Beta Breeding and Genetics at East Lansing, Michigan: Molecular Methods, Genetic Diversity, and Trait Elucidation. J. of Sugar Beet Research (USA),37(4): 97–106.
Morrison, R.A. and Evans, D.A. (1987). Gametoclonal variation. Plant breeding Reviews.5: 359–391.
Nei, M. and W.H., Li (1979). Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Nat Acad. Sci. USA,76(10): 5269–5273.
Srivastava, H.M. (1994). Pre breeding of Sugarbeet in India. Jour of Sugarbeet Research (USA),32: 99–111.
Srivastava, H.M. (1996). Genetic Diversity for High Temparature tolerance in diploid varieties of Sugarbeet. Proceedings of 4th InternationalBeta Genetic Resources Conference and WorldBeta Network meeting. A Report. International Crop Network Series No. 12, IPGRI (Rome) Eds: Frese, L, L. Panella H.M. Srivastava, W. Lange.
Svirshchevskaya, A.M., Borzyak, V.S. and Pryadko, A.V. (1997). Characteristics of Productivity traits in gynogenetic sugar beet regenerants. Izvestiya Akad. Nauk Belarusi, Ser. biol. nauk,1: 44–48 (in Russian).
Svirshchevskaya, A.M. and Borzyak V.S. (1998). Doubled haploids in sugar beet : spontaneous and colchicine-induced polypoidization.In : Current Topics in Plant Cytogenetics Related to Plant Improvement. Proc. Int. Symp., Wien Univ-Verl. p. 318–324.
Svirshchevskaya, A. and Dolezel J. (2000). Production and Performance of Gynogenetic Sugarbeet lines. J. of Sugar Beet Research (USA),37(4): 117–133.
Vos, P., Hogers, R., Bleeker, M., Reijans, M., van de Lee, T., Hornes Frijters, A., Pot, J., Pelentan, J., Kuiper, M.M. and Zabeau, M. (1995). AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research23(21) : 4407–4414.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Svirshchevskaya, A., Srivastava, H.M. Broadening of genetic base in sugar crops. Sugar Tech 3, 18–22 (2001). https://doi.org/10.1007/BF02945525
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02945525