Abstract
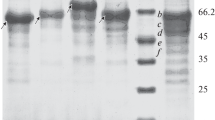
In order to study the secretion of the human urokinase-type plasminogen activator, u-PA, from the yeastYarrowia lipolytica, three kinds of integrative expression vector were constructed. These vectors differed only in their secretion control regions, pre-, pre-dip- (dipeptide stretch) or pre-dip-pro sequences of the alkaline extracellular protease, which were joined inframe to the human u-PA cDNA. The recombinantY. lipolytica strains, transformed with the expression vectors, secreted the hyperglycosylated u-PA. A fibrin plate assay of the culture supernatants showed that the hyperglycosylated u-PA proteins could catalyze fibrinolysis, and that the pre-dip sequence was the most efficient secretory signal for the secretion of the u-PA fromY. lipolytica. This result suggests thatY. lipolytica can be developed as a potential host for the production of recombinant human u-PA.
Similar content being viewed by others
References
Faber, K. N., W. Harder, G. Ab, and M. Veenhuis (1995) Review: methylotrophic yeasts as factories for the production of foreign proteins.Yeast 11: 1331–1344.
Ngsee, J. K. and M. Smith (1990) Changes in a mammalian signal sequence required for efficient protein secretion by yeasts.Gene 86: 251–255.
Schwientek, T. and J. F. Ernst (1994) Efficient intra- and extracellular production of human beta-1,4-galactosyltransferase inSaccharomyces cerevisiae is mediated by yeast secretion leaders.Gene 145: 299–303.
Barth, G. and C. Gaillardin (1997) Physiology and genetics of the dimorphic fungusYarrowia lipolytica.FEMS Microbiol. Rev. 19: 219–237.
Juretzek, T., M. Le Dall, S. Mauersberger, C. Gaillardin, G. Barth, and J. Nicaud (2001) Vectors for gene expression and amplification in the yeastYarrowia lipolytica.Yeast 18: 97–113.
Park, C. S., C. C. Chang, J. Y. Kim, D. M. Ogrydziak, and D. D. Ryu (1997) Expression, secretion, and processing of rice alpha-amylase in the yeastYarrowia lipolytica.J. Biol. Chem. 272: 6876–6881.
Pignede, G., H. Wang, F. Fudalej, C. Gaillardin, M. Seman, and J. M. Nicaud (2000) Characterization of an extracellular lipase encoded byLIP2 inYarrowia lipolytica.J. Bacteriol. 182: 2802–2810.
Chang, C. C., D. D. Ryu, C. S. Park, and J.-Y. Kim (1998) Improvement of heterologous protein productivity using recombinantYarrowia lipolytica and cyclic fed-batch process strategy.Biotechnol. Bioeng. 59: 379–385.
Park, C. S., J.-Y. Kim, C. Crispino, C. C. Chang, and D. D. Ryu (1998) Molecular cloning ofYIPMRI, aS. cerevisiae PMR1 homologue encoding a novel P-type secretory pathway Ca2−-ATPase, in the yeastYarrowia lipolytica.Gene 206: 107–116.
Kim, J. W., T. J. Park, D. D. Ryu, and J.-Y. Kim (2000) High cell density culture ofYarrowia lipolytica using a one-step feeding process.Biotechnol. Prog. 16: 657–660.
Pagot, Y., A. Endrizzi, J. M. Nicaud, and J. M. Belin (1997) Utilization of an auxotrophic strain of the yeastYarrowia lipolytica to improve gamma-decalactone production yields.Lett. Appl. Microbiol. 25: 113–116.
Pagot, Y., A. Le Clainche, J. M. Nicaud, Y. Wache, and J. M. Belin (1998) Peroxisomal beta-oxidation activities and gamma-decalactone production by the yeastYarrowia lipolytica.Appl. Microbiol. Biotechnol. 49: 295–300.
Lijnen, H. R. and D. Collen (1991) Strategies for the improvement of thrombolytic agents.Thromb. Haemost. 66: 88–110.
Wun, T. C., L. Ossowski, and E. Reich (1982) A proenzyme form of human urokinase.J. Biol. Chem. 257: 7262–7268.
Holmes, W. E., D. Pennica, M. Blaber, M. W. Rey, W. A. Guenzler, G. J. Steffens, and H. L. Heyneker (1985) Cloning and expression of the gene for pro-urokinase inEscherichia coli.Bio/Technology 3: 923–929.
Melnick, L. M., B. G. Turner, P. Puma, B. Price-Tillotson, K. A. Salvato, D. R. Dumais, D. T. Moir, R. J. Broeze, and G. C. Avgerinos (1990) Characterization of a nonglycosylated single chain urinary plasminogen activator secreted from yeast.J. Biol. Chem. 265: 801–807.
Hiramatsu, R., S. Horinouchi, and T. Beppu (1991) Isolation and characterization of human pro-urokinase and its mutants accumulated within the yeast secretory pathway.Gene 99: 235–241.
Tsujikawa, M., K. Okabayashi, M. Morita, and T. Tanabe (1996) Secretion of a variant of human single-chain urokinase-type plasminogen activator without an N-glycosylation site in the methylotrophic yeast,Pichia pastoris and characterization of the secreted product.Yeast 12: 541–553.
Agaphonov, M. O., A. N. Packeiser, M. B. Chechenova, E. S. Choi, and M. D. Ter-Avanesyan (2001) Mutation of the homologue of GDP-mannose pyrophosphorylase alters cell wall structure, protein glycosylation and secretion inHansenula polymorpha.Yeast 18: 391–402.
Nelles, L., H. R. Lijnen, D. Collen, and W. E. Holmes (1987) Characterization of recombinant human single chain urokinase-type plasminogen activator mutants produced by site-specific mutagenesis of lysine 158.J. Biol. Chem. 262: 5682–5689.
Ogrydziak, D. M. and R. K. Mortimer (1977) Genetics of extracellular protease production inSaccharomycopsis lipolytica.Genetics 87: 621–632.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryu, H.M., Kang, W.K., Kang, H.A. et al. Secretion of active urokinase-type plasminogen activator from the yeastYarrowia lipolytica . Biotechnol Bioproc E 8, 162–165 (2003). https://doi.org/10.1007/BF02940274
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02940274