Abstract
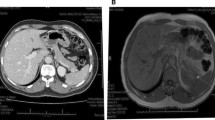
A case of acinar-islet cell carcinoma presenting as insulinoma is reported. The patient was a 28-year-old man who presented with two convulsive episodes. Fajans' index [immunoreactive insulin (IRI; μU/ml/ glucose mg/dl)] and Turner's [IRI (μU/ml) × 100/glucose (mg/dl)—30] index were high (2.8 and 308, respectively), as were serum proinsulin levels (550pg/ml). Abdominal computed tomography and angiography revealed a highly vascular tumor in the pancreatic tail and several similar tumors in the liver. Histologic features of a biopsy specimen from a hepatic tumor were those of a malignant pancreatic endocrine tumor. Insulin secretion by the liver metastases was confirmed by venous sampling after arterial stimulation with calcium. These findings led us to diagnose malignant insulinoma with liver metastases. Serum levels of α-fetoprotein and trypsin were markedly elevated, to 2234ng/ml (normal <10) and 22000ng/ml (normal<460) respectively, and these levels continued to rise with further growth of the liver metastases. Immunohistochemically, the metastatic liver tumor specimen was positive for α-fetoprotein, α1-antichymotrypsin, chromogranin A, and neuron-specific enolase. These findings of amphicrine features in the tumor were indicative of acinar-islet cell carcinoma that produced α-fetoprotein and trypsin in addition to insulin.
Similar content being viewed by others
References
Ulich T, Cheng L, Lewin KJ. Acinar-endocrine cell tumor of the pancreas. Report of a pancreatic tumor containing both zymogen and neuroendocrine granules. Cancer 1982;50:2099–2105.
Ichijima K, Akaishi K, Toyoda N, et al. Carcinoma of the pancreas with endocrine component in childhood. A case report. Am J Clin Pathol 1985;83:95–100.
Machida H, Nakaya Y, Kojima K, et al. A case of acinar-islet cell tumor of the pancreas body (in Japanese with English abstract). Nippon Geka Gakkai Zasshi (J Jpn Surg Soc) 1991;92:603–606.
Klimstra DS, Rosai J, Heffess CS. Mixed acinar-endocrine carcinomas of the pancreas. Am J Surg Pathol 1994;18:765–778.
Sommers SC, Meissner WA. Unusual carcinomas of the pancreas. AMA Arch Pathol 1954;58:101–111.
Glenner GG, Mallory GK. The cystadenoma and related nonfunctional tumors of the pancreas. Pathogenesis, classification, and significance. Cancer 1956;9:980–996.
Schron DS, Mendelsohn G. Pancreatic carcinoma with duct, endocrine, and acinar differentiation. A histologic, immunocytochemical, and ultrastructural study. Cancer 1984;54:1766–1770.
Nonomura A, Kono N, Mizukami Y, et al. Duct-acinar-islet cell tumor of the pancreas. Ultrastruct Pathol 1992;16:317–329.
Hassan MO, Gogate PA. Malignant mixed exocrine-endocrine tumor of the pancreas with unusual intracytoplasmic inclusions Ultrastruct Pathol 1993;17:483–493.
Okada Y, Mori H, Tsutsumi A. Duct-acinar-islet cell tumor of the pancreas. Pathol Int 1995;45:669–676.
Fajans SS, Floyd JC. Fasting hypoglycemia in adults. N Engl J Med 1976;294:766–772.
Turner RC, Oakley NW, Nabarro JDN. Control of basal insulin secretion, with special reference to the diagnosis of insulinomas. BMJ 1971;2:132–135.
Doppman JL, Miller DL, Change R, et al. Insulimomas: Localization with selective intraarterial injection of calcium. Radiology 1991;178:237–241.
Lee PC, Lebenthal E. Prenatal and postnatal development of the human exocrine pancreas. In: Go VLW, Dimagno EP, Gardner JD, Lebenthal E, Reber HA, Sheele GA (eds). The pancreas. New York: Raven, 1993:57–73.
Githens S. Differentiation and development of the pancreas in animals. In: Go VLW, Dimagno, EP, Gardner JD, Lebenthal E, Reber HA, Sheele GA (eds). The pancreas. New York: Raven, 1993:21–55.
Kodama T, Mori W. Morphological behavior of carcinoma of the pancreas. 2. Argyrophil cells and Langerhans' islets in the carcinomatous tissues. Acta Pathol Jpn 1983;33:483–493.
Kay D, Delellis RA, Dayal Y, et al. Ductal adenocarcinoma of the pancreas with neuroendocrine cells: An immunohistochemical study. Lab Invest 1985;52:33–34A.
Klimstra DS, Heffess CS, Oertel, JE. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol 1992;16:815–837.
Inaba N, Kasahara K, Kashii A, et al., Mixed ductal and acinar cell cancer of the pancreas head; report of a case (in Japanese with English abstract). Nippon Geka Gakkai Zasshi (J Jpn Surg Soc) 1987;88:773–777.
Tomita T, Bhatia P, Gourley W. Mucin producing islet cell adenoma. Hum Pathol 1981;12:850–853.
Reid JD, Yuh SL, Petrelli M, et al. Ductuloinsular tumors of the pancreas: A light, electron microscopic and immunohistochemical study. Cancer 1982;49:908–915.
Arihori K, Inai K. Malignant islet cell tumor of the pancreas with multiple hormone production and expression of CEA and CA19-9. Report of an autopsy case. Acta Pathol Jpn 1991;41:150–157.
Kashiwabara K, Nakajima T, Shinkai H, et al. A case of malignant duct-islet cell tumor of the pancreas: Immunohistochemical and cytofluorometric study. Acta Pathol Jpn 1991;41:636–641.
Gitlin D, Perricelli A, Gitlin GM. Synthesis of alpha-fetoprotein by liver, yolk sac and gastrointestinal tract of the human conceptus. Cancers Res 1972;32:979–982.
Kawamoto S, Hiraoka T, Kanemitsu K, et al. Alpha-fetoprotein-producing pancreatic cancer—a case report and review of 28 cases. Hepatogastroenterology 1992;39:282–286.
Kato K, Akai S, Tobita Y, et al. α-Fetoprotein-positive cases in cancer exept for hepatoma and malignant teratoma—review of data in Japan. Jpn J Cancer Clin 1974;20:376–382.
Hoorens A, Lemonie NR, McLellan E, et al., Pancreatic acinar cell carcinoma. An analysis of cell lineage markers, p53 expression, and ki-ras mutation. Am J Pathol 1993;143:685–698.
Kawai S, Watanabe, K, Hirama S, et al. An alpha-fetoprotein-producing pancreatic carcinoma (in Japanese with English abstract). Jpn J Cancer Clin 1992;38:1013–1018.
Nojima T, Kojima T, Kato H, et al. Alpha-fetoprotein-producing acinar cell carcinoma of the pancreas. Hum Pathol 1992;23:828–830.
Shinagawa T, Tadokoro T, Maeyama S, et al. Alpha fetoprotein-producing acinar cell carcinoma of the pancreas showing multiple lines of differentiation. Virchows Arch 1995; 426:419–423.
Shimizu E, Kikuyama M, Hashimoto M, et al. A case of α-fetoprotein-producing pancreatic carcinoma. Review of 41 cases reported in Japan (in Japanese). Jpn J Gastroenterol 1996;93:921–926.
Yasuo E. Carcino-fetal antigens (in Japanese with English abstract). Nippon Rinsho 1996;54:1499–1504.
Ishiguro T, Yoshida Y. Molecular heterogenecity of alphafetoprotein in ovarian or extragonadal yolk sac tumor (in Japanese with English abstract). Acta Obstet Gynaecol Jpn 1991;43:391–398.
Fukuda H, Yano M, Houchi N, et al. A, case of nonseminomatous germ cell tumor: Usefulness of alphafetoprotein subfracton (in Japanese with English abstract). Hinyokika-Kiyo (Acta Urol Jpn) 1994;40:357–360.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shimoike, T., Goto, M., Nakano, I. et al. Acinar-islet cell carcinoma presenting as insulinoma. J Gastroenterol 32, 830–835 (1997). https://doi.org/10.1007/BF02936964
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02936964