Abstract
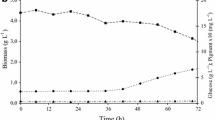
For the purpose of mass producingMonascus red pigments optimum medium composition and environmental conditions were investigated in submerged flask cultures. The optimum carbon and nitrogen sources were determined to be 30 g/L of glucose and 1.5 g/L of monosodium glutamate (MSG). Of the three metals examined, Fe2+ showed the stronges stimulatory effect on pigment production and some stimulatory effect was also found in Mn2+. Optimum pH and agitation speed were determined to be 6.5 and 700 rpm, respectively. Under the optimum culture conditions batch fermentation showed that the maximum biomass yield and specific productivity of red pigments were 0.20 g DCW/g glucose and, 32.5 OD500 g DCW−1 h−1, respectively.
Similar content being viewed by others
References
Lauro, G. J. (1991) A primer on natural colors.Cereal Food World 36: 949–953.
Wong, H. C. and P. E. Koeheler (1983) Production of red water solubleMonascus pigments.J. Food Sci. 48: 1200–1203.
Yoshimura, M., S. Yamanaka, K. Mitsugi, and Y. Hirose (1975) Production ofMonascus pigment in a submerged culture.Agr. Biol. Chem. 39: 1789–1795.
Ohshima, M., N. Shizaki, and Y. Tonooka (1985) Production of neuropurpuratin, purplish-red pigment, by pure culture ofStreptomyces propurpuratus.J. Ferment. Technol. 63: 79–83.
Trias, J., M. Vinas, J. Guinea, and J. G. Loren (1988) Induction of yellow pigmentation inSerratia marcescens.Appl. Environ. Microbiol. 54: 3138–3141.
Lin, C. E. (1973) Isolation and culture condition ofMonascus sp. for the production of gigment in submerged culture.J. Ferment. Technol. 51: 407–414.
Pastrana, L., P. C. Blanc, M. O. Santerre, O. Loret, and G. Goma (1995) Production of red pigments byMonascus ruber in synthetic media with a strictly controlled nitrogen source.Process Biochem. 30: 333–341.
Wang, H. L. and C. W. Hesseltine (1979) Mold-modified foods. pp. 95–129. In: H. J. Peppler and J. Perlman (eds.).Microbial Technology. Academic Press, New York, USA.
Chen, M. H. and M. R. Johns (1993) Effect of pH and nitrogen source on pigment production byMonascus purpureus.Appl. Microb. Biotechnol. 40: 132–138.
Chen, M. H. and M. R. Johns (1994) Effect of carbon source on ethanol and pigment production byMonascus purpureus.Enzyme. Microb. Technol. 16: 584–590.
Juzlova, P., I. Martincova, and J. Lozinski (1994) Ethanol as substrate for pigment production by the fungusMonascus.Enzyme Microb. Technol. 16: 996–1001.
Miller, G. L. (1959) Use of dinitosalicylic acid regent for determination of reducing sugar.Anal. Chem. 31: 426–428.
Lin, T. F., K. B. Yakushijin, G. H. Chi, and A. L. Demain (1991) Formation of water-solubleMonascus red pigments by biological and semi-synthetic processes.J. Indust. Microbiol. 9: 173–179.
De Deken, H. (1966) The Crabtree effect: A regulatory system in yeast.J. Gen. Microbiol. 44: 149–156.
Spepherd, D. (1977) The relationship between pigment production and sporulation inMonascus. pp. 102–118. In: J. Meyrath and J. D. Bulock (eds.).Biotechnolgy and Fungal Differentiation. Academic Press, London, UK.
Broder, C. U. and P. E. Koehler (1980) Pigment production byMonascus purpureous with regard to quality and quantity.J. Food. Sci. 45: 567–569.
Carels, M. and D. Shepherd. (1977) The effect of different nitrogen sources on pigment production and sporulation ofMonascus species in submerged shaken culture.Can. J. Microbiol. 23: 1360–1372.
Juzlova, P., L. Martinkova, and V. Kren (1996) Secondary metabolites of the fungusMonascus: A review.J. Indust. Microbiol. 16: 163–170.
Bau, Y. S. and H. C. Wong (1979) Zinc effects on growth pigmentation and antibacterial activity ofMonascus purpureous.Physiol. Plant. 46: 63–67.
Weinberg, E. D. (1989) Roles of micronutrients in secondary metabolism ofActinomycetes. pp. 239–261. In: Shapiro, S. (ed.).Regulation of Secondary Metabolism in Actinomycetes. CRC Press, Boca Raton, USA.
Takahashi, J., H. Hidaka, and K. Yamada (1965) Effect of mycelial forms on citric acid fermentation in submerged mold culture.Agr. Biol. Chem. 29: 331–336.
Su, Y. C. and J. H. Huang (1980) Fermentative production of anka-pigments (Monascus-pigments).Proc. Natl. Sci. Counc. ROC 4: 201–215.
Han, S. and R. E. Mudgen (1992) Effects of oxygen and carbon dioxide partial pressures onMonascus growth and pigment production in solid-state fermentations.Biotechnol. Prog. 8: 5–10.
Turner, W. B. (1971).Fungal Metabolites. 2nd ed., pp. 136–139. Acddemic Press, London, UK.
Johns, M. R., R. Chong, and I. S. Maddox (1982) Hydrolysis of some natural and synthetic bile acid conjugates byCerospora melonis.Can. J. Microbiol. 28: 457–461.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, BK., Park, NH., Piao, H.Y. et al. Production of red pigments byMonascus purpureus in submerged culture. Biotechnol. Bioprocess Eng. 6, 341–346 (2001). https://doi.org/10.1007/BF02933003
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02933003