Abstract
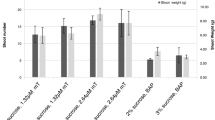
Among the various plant growth regulators (PGR), 9.0 μM 2,4-D(2,4-dichlorophenoxyacetic acid) turned out to be the best for callogenesis and 4.5 μM 2,4-D was revealed to be more suitable for callus proliferation. Of the four basal media, the highest number of 8.2 adventitious roots was induced in Woody plant medium (WPM) containing 14.8 μM IBA(indole 3-butyic acid). F tests on each auxin treatment in the ANOVA revealed highly significant difference at α=0.01. The number of multiple adventitious roots (MARs) formed in each adventitious roots highly depended on the types and levels of auxin tested. At both 24.6 μM and 34.4 μM IBA, more than 25 MARs were induced after 4 weeks of culture. When different medium were applied, immersion type showed the highest fresh weight of MARs at the time of harvest. With 160 g fresh weight of inoculum, about 14.2 times of biomass was obtained after 6 weeks of culture. Identical fractionation pattern of the amplified fragment length polymorphism (AFLP) analysis was observed between the adventitious root and donor of a mountain ginseng. This observation suggested that any rearrangements of the genomic DNA had not occurred in the adventitious roots after 1 year of cultures. The cultivated ginseng and the mountain ginseng groups could be distinguished by 2 AFLP markers.
Similar content being viewed by others
References
Nishi, A. (1994) Effect of elicitors on the production of secondary metabolites pp. 95–102 In:Advances in Plant Biotechnology. Elsevier Science B. V. Publisher.
Ballica, R. and D. Y. Ryu (1994) Tropane alkaloid production fromDatura stramonium: An integrated approach to bioprocess optimization of plant cell cultivation pp. 221–254 In: N.-H. Chua, E. Conn, M. L. Shuler, Y. Yamada, and Meinhard (eds.)Advances in plant biotechnology. Elsevier Science B. V. Netherlands.
Kurata, H., M. Seki, and S. Furusaki (1994) Light effect to promote secondary metabolite production of plant cell culture. pp. 103–133 In: N.-H. Chua, E. Conn, M. L. Shuler, Y. Yamada, and Meinhard (eds.)Advances in plant biotechnology. Elsevier Science B. V. Netherlands.
Zhong, J.-J. and T. Yoshda (1994) Rheological characteristics of suspended cultures ofPerilla frutescens and their implications in bioreactor operating for anthocyanin production. In: N.-H. Chua, E. Conn, M. L. Shuler, Y. Yamada, and Meinhard (eds.)Advances in plant biotechnology. Elsevier Science B. V. Netherlands.
Yamada, Y. and Y. Fujita (1983) Production of useful compounds in culture. pp. 717–728 In: D. A. Evans, W. R. Sharp, P. V. Ammirato, and Y. Yamada (eds.)Handbook of plant cell culture techniques for propagation and breeding. Vol. 1. Macmillan Publishing Co. New York.
Payane, G., V. Bringi, C. Prince, and M. L. Shuler (1991) The quest for commercial production of chemicals from plant cell culture. pp. 1–10 In: M. L. Shuler (eds.)Plant cell and tissue culture in liquid systems. Carl Hanser Verlag. Hanser.
Flores, H. E., M. W. Hoy, and J. J. Pickard (1987) Secondary metabolites from root cultures.Trends Biotechnol. 5: 64–69.
Aird, E. L. H., J. D. Hamil, R. J. Robins, and M. J. C. Rhodes (1988) Chromosome stability in transformed hairy root cultures and the properties of variant lines ofNicotina rustica hairy roots. pp. 137–144 In: R. J. Robins and M. J. C. Rhodes (eds.)Manipulating secondary metabolism in culture. Cambridge University Press. Cambridge, U.K.
Toppel, G., L. Witte, B. Riebeseh, K. V. Borstel, and T. Hartmann (1987) Alkaloid patterns and biosynthetic capacity of root cultures from some pyrrolizidine alkaloid producingsenecio species.Plant Cell Rep. 6: 466–469.
Berlin, J., V. Way, C. Mollenshott, and F. Sasse (1988) Formation of β-peltatin-a methyl esther and coniferin by root cultures ofLinum flavum.J. Nat. Prod. 49: 435–439.
Hashimoto, T. and Y. Yamada (1983) Scopolamine production in suspension cultures and redifferentiated roots ofHyoscyamus niger.J. Med. Plant Res. 47: 195–199.
Ko, K. M., J. J. Song, B. Hwang, and Y. H. Kang (1993) Cytogenetic and historical characteristic of ginseng hairy root transformed byAgrobacterium rhizogenes.Kor. J. Bot. 36(1): 75–81.
Yoshikawa, T. and T. Furuya (1987) Saponin production by cultures ofPanax ginseng transformed withAgrobaterium rhizonenes.Plant Cell Rep. 6: 449–453.
Murashige, T. and F. Skoog (1962) Revised medium for rapid growth and bioassay with tobacco tissue culture.Physiol. Plant 15: 473–479.
Lloyd, G. B. and B. H. McCown (1981) Commercially feasible micro-propagation of mountain laurel (Kamlmia latifolia) by use of shoot tip culture.Prop. Int. Plant Proc. Soc. 30: 421–427.
Schenk, R. U. and A. C. Hildebrandt (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures.Can. J. Bot. 50: 199–204.
White, P. (1934) Potentially unlimited growth of excised tomato root tips in a liquid medium.Plant Physiol. 9: 585–600.
Hong, Y.-P. (1991) Chloroplast DNA variability and phylogeny in the California closed cone pines.Ph.D. Thesis. Oregon State University.
Zabeau, M. and P. Vos (1993) Selective restriction fragment amplification: a general method for DNA fingerprinting.European Pat. Appl. 92402629.7 (Publ. No. 0 534 858 A1).
Hongtrakul, V., G. M. Husetis, and S. J. Knapp (1997) Amplified fragment length polymorphisms as a tool for DNA fingerprinting sunflower germplasm: genetic diversity among oil-seed inbred lines.Theoretical Applied Genetics. 95: 400–407.
Son, S. H. and R. B. Hall (1990) Plant regeneration capacity of callus derived from leaf, stem, and root segments ofPopulus alba L.×P. grandidentata Michx.Plant Cell Rep. 9: 344–347.
Inomata, S., M. Yokoyama, Y. Gozu, T. Shimizu, and M. Yanagi (1993) Growth pattern and ginsennoside production ofAgrobaterium-transformedPanax ginseng roots.Plant Cell Rep. 12: 681–686.
Carrier, D. J., G. Cosentino, R. Neufeld, D. Rho, M. Weber, and J. L. Archambault (1990) Nutritional and hormonal requirements ofGinkgo biloba embryo-derived callus and suspension cell culture.Plant Cell Rep. 8: 635–638.
Furuya, T., T. Yoshikawa, Y. Orihara, and H. Oda (1983) Saponin production in cell suspension cultures ofPanax ginsneg.Planta Med. 48: 83–87.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Son, S.H., Choi, S.M., Hyung, S.J. et al. Induction and cultures of mountain ginseng adventitious roots and AFLP analysis for identifying mountain ginseng. Biotechnol. Bioprocess Eng. 4, 119–123 (1999). https://doi.org/10.1007/BF02932381
Issue Date:
DOI: https://doi.org/10.1007/BF02932381