Abstract
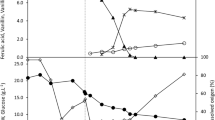
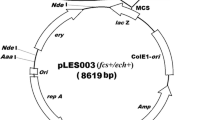
Vanillin is one of the world's principal flavoring compounds, and is used extensively in the food industry. The potential vanillin production of the bacteria was compared to select and clone genes which were appropriate for highly productive vanillin production byE. coli. Thefcs (feruloyl-CoA synthetase) andech (enoyl-CoA hydratase/aldolase) genes cloned fromAmycolatopsis sp. strain HR104 andDelftia acidovorans were introduced to pBAD24 vector with PBAD promoter and were named pDAHEF and pDDAEF, respectively. We observed 160 mg/L vanillin production withE. coli harboring pDAHEF, whereas 10 mg/L of vanillin was observed with pDDAEF. Vanillin production was optimized withE. coli harboring pDAHEF. Induction of thefcs andech genes from pDAHEF was optimized with the addition of 13.3 mM arabinose at 18 h of culture, from which 450 mg/L of vanillin was produced. The feeding time and concentration of ferulic acid were also optimized by the supplementation of 0.2% ferulic acid at 18 h of culture, from which 500 mg/L of vanillin was obtained. Under the above optimized condition of arabinose induction and ferulic acid supplementation, vanillin production was carried out with four different types of media, M9, LB, 2YT, and TB. The highest vanillin production, 580 mg/L, was obtained with LB medium, a 3.6 fold increase in comparison to the 160 mg/L obtained before the optimization of vanillin production.
Similar content being viewed by others
References
Krings, U. and R. G. Berger (1998) Biotechnological production of flavours and fragrances.Appl. Microbiol. Biotechnol. 49: 1–8.
Lomascolo, A., C. Stentelaire, M. Asther, and L. Lesage-Meessen (1999) Basidiomycetes as new biotechnological tools to generate natural aromatic flavours for the food industry.Trends Biotechnol. 17: 282–289.
Lopez-Malo, A., S. Alzamora, and A. Agraiz (1995) Effect of natural vanillin on germination time and radial growth rate of moulds in fruit agar systems.Food Microbiol. 12: 215–219.
Cerrutti, P. and S. M. Alzamora (1996) Inhibitory effects of vanillin on some food spoilage yeasts in laboratory mecia and fruit purees.Int. J. Food. Microbiol. 29: 379–386.
Cerrutti, P. S., M. Alzamora, and S. Vidales (1997) Vanillin as an antimicrobial for producing shelf-stable strawberry puree.J. Food Sci. 62: 608–610.
Burri, J., M. Graf, P. Lambelet, and J. Loiger (1989) Vanillin: More than a flavouring agent-a potent antioxidant.J. Sci. Food. Agric. 48.
Prince, R. C. and D. E. Gunson (1994) Just plain vanilla?Trends Biochem. Sci. 19: 521.
Lesage-Meesen, L., M. Delattre, M. Haon, J. F. Thibault, B. C. Ceccaldi, P. Brunerie, and M. Asther (1996) A two-step bioconversion process for vanillin production from ferulic acid combiningAspergillus niger andPycnoporus cimabarinus.J. Biotechnol. 50: 107–113.
Cheetham, P. S. J. (1993) The use of biotransformations for the production of flavours and fragrances.Trends Biotechnol. 11: 478–488.
Hagedorn, S. and B. Kaphammer (1994) Microbial biocatalysis in the generation of flavor and fragrance chemicals.Ann. Rev. Microbiol. 48: 773–800.
Overhage, J., H. Priefert, J. Rabenhorst, and A. Steinbuchel (1999) Biotransformation of eugenol to vanillin by a mutant ofPseudomonas sp. strain HR199 constructed by disruption of the vanillin dehydrogenase (vdh) gene.Appl. Microbiol. Biotechnol. 52: 820–828.
Li, T. and J. P. Rosazza (2000) Biocatalytic synthesis of vanillin.Appl. Environ. Microbiol. 66: 684–687.
Walton, N. J., A. Narbad, C. Faulds, and G. Williamson (2000) Novel approaches to the biosynthesis of vanillin.Curr. Op. Biotechnol. 11: 490–496.
Muheim, A. and K. Lerch (1999) Towards a high-yield bioconversion of ferulic acid to vanillin.Appl Microbiol. Biotechnol. 51: 456–461.
Achterholt, S., H. Priefert, and A. Steinbuchel (2000) Identification ofAmycolatopsis sp. strain HR167 genes, involved in the bioconversion of ferulic acid to vanillin.Appl. Microbiol Biotechnol. 54: 799–807.
Peng, X., N. Misawa, and S. Harayama (2003) Isolation and characterization of thermophilic bacilli degrading cinnamic, 4-coumaric, and ferulic acids.Appl. Environ. Microbiol. 69: 1417–1427.
Plaggenborg, R., A. Steinbuchel, and H. Priefert (2001) The coenzyme A-dependent, non-beta-oxidation pathway and not direct deacetylation is the major route for ferulic acid degradation inDelftia acidovorans.FEMS Microbiol. Lett. 205: 9–16.
Plaggenborg, R., J. Overhage, A. Steinbuchel, and H. Priefert (2003) Functional analyses of genes involved in the metabolism of ferulic acid inPseudomonas puida KT2440.Appl. Microbiol. Biotechnol. 61: 528–535.
Masai, E., K. Harada, X. Peng, H. Kitayama, Y. Katayama, and M. Fukuda (2002) Cloning and characterization of the ferulic acid catabolic genes ofSphingomonas paucinobilis SYK-6.Appl. Environ. Microbiol. 68: 4416–4424.
Sutherland, J. B., D. L. Crawford, and A. L. Pometto 3rd (1983) Metabolism of cinnamic,p-coumaric, and ferulic acids byStreptomyces setonii.Can. J. Microbiol. 29: 1255–1257.
Rabenhorst, J. and R. Hopp (1997) Verfahren zur Herstellung von Vanillin und dafur geeignete Mikroorganismen.German Patent EP0761817A2.
Muheim, A., B. Muller, T. Munch, and M. Wetli (1998) Process for the production of vanillin.German Patent EP0885968A1.
Choi, J. I., S. Y. Lee, K. S. Shin, W. G. Lee, S. J. Park, H. N. Chang, and Y. K. Chang (2002) Pilot scale production of poly(3-hydroxybutyrate-co-3-hydroxy-valerate) by fedbatch culture of recombinantEscherichia coli.Biotechnol. Bioprocess Eng. 7: 371–374.
Choi, J. I. and S. Y. Lee (2004) High level production of supra molecular weight poly(3-hydroxybutryrate) by metabolically engineeredEscherichia coli.Biotechnol. Bioprocess Eng. 9: 196–200.
Kim, J. Y. and D. Y. Ryu (1999) Physiological and environmental effects on metabolic flux change caused by heterologous gene expression inEscherichia coli.Biotechnol. Bioprocess Eng. 4: 170–175.
Hopwood, D. A., M. J. Bibb, K. F. Chater, T. Kieser, C. J. Bruton, H. M. Kieser, D. J. Lydiate, C. P. Smith, J. M. Simth, J. M. Ward, and H. S. Schrempf (1985)Genetic Manipulation of Streptomycetes: A Laboratory Manual. John Innes Institute, Norwich, UK.
Sambrock, J. and D. W. Russell (2001)Molecular Cloning. 2nd ed., Cold Spring Harbor Laboratory Press, NY, USA.
Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith (1995) Hight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter.J. Bacteriol. 177: 4121–4130.
Gasson, M. J., Y. Kitamura, W. R. Mclauchlan, A. Narbad, A. J. Parr, E. L. Parsons, J. Payne, M. J. Rhodes, and N. J. Walton (1998) Metabolism of ferulic acid to vanillin. A bacterial gene of the enoyl-SCoA hydratase/isomerase superfamily encodes an enzyme for the hydration and cleavage of a hydroxycinnamic acid SCoA thioester.J. Biol. Chem. 275: 4163–4170.
Andreoni, V., E. Galli, and G. Galliani (1984) Metabolism of ferulic acid by a facultatively anaerobic strain ofPseudomonas cepacia.Syst. Appl. Microbiol. 5: 299–304.
Otuk, G. (1985) Degridation of ferulic acid byEscherichia coli.J. Ferment. Technol. 63: 501–506.
Gurujeyalakshmi, G. and A. Mahadevan (1987) Disimilation of ferulic acid byBacillus subtilis.Curr. Microbiol. 16: 69–73.
Matamoros-Leon, B., A. Argaiz, and A. Lopez-Malo (1999) Individual and combined effects of vanillin and potassium sorbate onPenicillium digitatum. Penicillium glabrum, andPenicillium italicum growth.J. Food Prot. 62: 540–542.
Zaldivar, J., A. Martinez, and L. O. Ingram (1999) Effect of selected aldehydes on the growth and fermentation of ethanologenicEscherichia coli.Biotechnol. Bioeng. 65: 24–33.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, SH., Li, C., Lee, YM. et al. Production of vanillin from ferulic acid using recombinant strains ofEscherichia coli . Biotechnol. Bioprocess Eng. 10, 378–384 (2005). https://doi.org/10.1007/BF02931859
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02931859