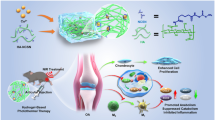
Abstract
Ultrasound (US) is being used widely in clinic for diagnostic and therapeutic purposes, but clinical utilization of low intensity ultrasound (LIUS) has been very limited. However, therapeutic potential of LIUS has been reported in animal models of musculoskeletal system disorders, and its application is being expanded in various fields. This review will focus on the application of LIUS on the cartilage tissue engineering and repair of cartilage disorder such as osteoarthritis (OA). We will introduce our experimental results showing the LIUS effects on the chondrocyte viability, proliferation and matrix protein synthesisin vitro, and its application in the cartilage tissue engineering using mesenchymal stem cells (MSCs)in vivo. Also the current status on the issues will be discussed by comparing our results with those of other laboratories. In conclusion, we suggest that LIUS is an efficient and clinically applicable method for cartilage tissue engineering and cartilage repair.
Similar content being viewed by others
References
Shung, K. K. (2004) Ultrasound and tissue interaction.Encycl. Biomat. Biomed. Eng. pp. 1706–1714.
Dalecki, D. (2004) Mechanical bioeffects of ultrasound.Annu. Rev. Biomed. Eng. 6: 229–248.
Merino, G., Y. N. Kalia, M. B. Delgado-Charro, R. O. Potts, and R. H. Guy (2003) Frequency and thermal effects on the enhancement of transdermal transport by sonophoresis.J. Control Release 88: 85–94.
Feril, L. B. Jr. and T. Kondo (2004) Biological effects of low intensity ultrasound: the mechanism involved, and its implications on therapy and on biosafety of ultrasound.J. Radiat. Res. 45: 479–489.
Liu, J., I. Sekiya, K. Asai, T. Tada, T. Kato, and N. Matsui (2001) Biosynthetic response of cultured articular chondrocytes to mechanical vibration.Res. Exp. Med. (Berl). 200: 183–193.
Parvizi, J., C.-C. Wu, D. G. Lewallen, J. F. Greenleaf, and M. E. Bolander (1999) Low-intensity ultrasound stimulates proteoglycan synthesis in rat chondrocytes by increasing aggrecan gene expression.J. Orthop. Res. 17: 488–494.
Deng, C. X., F. Sieling, H. Pan, and J. Cui (2004) Ultrasound-induced cell membrane porosity.Ultrasound Med. Biol. 30: 519–526.
Duarte, L. R. (1983) The stimulation of bone growth by ultrasound.Arch. Orthop. Trauma Surg. 101: 153–159.
Hadjiargyrou, M., K. McLeod, J. P. Ryaby, and C. Rubin (1998) Enhancement of fracture healing by low intensity ultrasound.Clin. Orthop. Relat. Res. 355 Suppl: S216-S219.
Heckman, J. D., J. P. Ryaby, J. McCabe, J. J. Frey, and R. F. Kilcoyne (1994) Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound.J. Bone Joint Surg. Am. 76: 26–34.
Pilla, A. A., M. A. Mont, P. R. Nasser, S. A. Khan, M. Figueiredo, J. J. Kaufman, and R. S. Siffert (1990) Non-invasive low-intensity pulsed ultrasound accelerates bone healing in the rabbit.J. Orthop. Trauma 4: 246–253.
Wiltink, A., P. J. Nijweide, W. A. Oosterbaan, R. T. Hekkenberg, and P. J. M. Helders (1995) Effect of therapeutic ultrasound on endochondral ossification.Ultrasound Med. Biol. 21: 121–127.
Rantanen, J., O. Thorsson, P. Wollmer, T. Hurme, and H. Kalimo (1999) Effects of therapeutic ultrasound on the regeneration of skeletal myofibers after experimental muscle injury.Am. J. Sports Med. 27: 54–59.
Enwemeka, C. S., O. Rodriguez, and S. Mendosa (1990) The biomechanical effects of low-intensity ultrasound on healing tendons.Ultrasound Med. Biol. 16: 801–807.
Cook, S. D., S. L. Salkeld, L. S. Popich-Patron, J. P. Ryaby, D. G. Jones, and R. L. Barrack (2001) Improved cartilage repair after treatment with low-intensity pulsed ultrasound.Clin. Orthop. Relat. Res. 391 Suppl: S231-S243.
Nieminen, H. J., S. Saarakkala, M. S. Laasanen, J. Hirvonen, J. S. Jurvelin, and J. Toyras (2004) Ultrasound attenuation in normal and spontaneously degenerated articular cartilage.Ultrasound Med. Biol. 30: 493–500.
Miller, M. W. (2000) Gene transfection and drug delivery.Ultrasound Med. Biol. 26 Suppl 1: S59-S62.
Wei, W., B. Zheng-zhong, W. Yong-jie, Z. Qing-wu, and M. Ya-lin (2004) Bioeffects of low-frequency ultrasonic gene delivery and safety on cell membrane permeability control.J. Ultrasound Med. 23: 1569–1582.
Kost, J. (1993) Ultrasound for controlled delivery of therapeutics.Clin. Mater. 13: 155–161.
Park, S. R., K. W. Jang, S.-H. Park, H. S. Cho, C. Z. Jin, M. J. Choi, S. I. Chung, and B.-H. Min (2005) The effect of sonication on simulated osteoarthritis. Part I: Effects of 1 MHz ultrasound on uptake of hyaluronan into the rabbit synovium.Ultrasound Med. Biol. 31: 1551–1558.
Mow, V. C., C. C. Wang, and C. T. Hung (1999) The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage.Osteoarthr. Cartil. 7: 41–58.
Hung, C. T., D. R. Henshaw, C. C. Wang, R. L. Mauck, F. Raia, G. Palmer, P. H. Chao, V. C. Mow, A. Ratcliffe, and W. B. Valhmu (2000) Mitogen-activated protein kinase signaling in bovine articular chondrocytes in response to fluid flow dose not require calcium mobilization.J. Biomech. 33: 73–80.
Frank, E. H. and A. J. Grodzinsky (1987) Cartilage electromechanics —II. A continuum model of cartilage electrokinetics and correlation with experiments.J. Biomech. 20: 629–639.
Garcia, A. M., E. H. Frank, P. E. Grimshaw, and A. J. Grodzinsky (1996) Contributions of fluid convection and electrical migration to transport in cartilage: relevance to loading.Arch. Biochem. Biophys. 333: 317–325.
Setton, L. A., V. C. Mow, and D. S. Howell (1995) Mechanical behavior of articular cartilage in shear is altered by transection of the anterior cruciate ligament.J. Orthop. Res. 13: 473–482.
Setton, L. A., V. C. Mow, F. J. Muller, J. C. Pita, and D. S. Howell (1997) Mechanical behavior and biochemical composition of canine knee cartilage following periods of joint disuse and disuse with remobilization.Osteoarthr. Cartil. 5: 1–16.
Grandolfo, M., A. Calabrese, and P. D'Andrea (1998) Mechanism of mechanically induced intercellular calcium waves in rabbit articular chondrocytes and in HIG-82 synovial cells.J. Bone Miner. Res. 13: 443–453.
Wright, M. O., K. Nishida, C. Bavington, J. L. Godolphin, E. Dunne, S. Walmsley, P. Jobanputra, G. Nuki, and D. M. Salter (1997) Hyperpolarisation of cultured human chondrocytes following cyclical pressure-induced strain: Evidence of a role for α5β1 integrin as a chondrocyte mechanoreceptor.J. Orthop. Res. 15: 742–747.
Zhang, Z.-J., J. Huckle, C. A. Francomano, and R. G. S. Spencer (2003) The effects of pulsed low-intensity ultrasound on chondrocyte viability, proliferation, gene expression and matrix production.Ultrasound Med. Biol. 29: 1645–1651.
Huang, M.-H., H.-J. Ding, C.-Y. Chai, Y.-F. Huang, and R.-C. Yang (1997) Effects of sonication on articular cartilage in experimental osteoarthritis.J. Rheumatol. 24: 1978–1984.
Choi, B. H., J.-I. Woo, B.-H. Min, and S. R. Park (2006) Low-intensity ultrasound (LIUS) stimulates the viability and matrix gene expression of human articular chondrocytes in alginate bead culture.J. Biomed. Mater. Res. A 79: 858–864.
Doan, N., P. Reher, S. Meghji, and M. Harris (1999)In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts, and monocytes.J. Oral Maxillofac. Surg. 57: 409–419.
Harle, J., V. Salih, F. Mayia, J. C. Knowles, and I. Olsen (2001) Effects of ultrasound on the growth and function of bone and periodontal ligament cellsin vitro.Ultrasound Med. Biol. 27: 579–586.
Tsai, W. C., C. C. Hsu, F. T. Tang, S. W. Chou, Y. J. Chen, and J. H. Pang (2005) Ultrasound stimulation of tendon cell proliferation and upregulation of proliferating cell nuclear antigen.J. Orthop. Res. 23: 970–976.
Nishikori, T., M. Ochi, Y. Uchio, S. Maniwa, H. Kataoka, K. Kawasaki, K. Katsube, and M. Kuriwaka (2002) Effects of low-intensity pulsed ultrasound on proliferation and chondroitin sulfate synthesis of cultured chondrocytes embedded in Atelocollagen gel.J. Biomed. Mater. Res. 59: 201–206.
Min, B.-H., J.-I. Woo, H.-S. Cho, B. H. Choi, S.-J. Park, M. J. Choi, and S. R. Park (2006) Effects of low-intensity ultrasound (LIUS) stimulation on human cartilage explants.Scand. J. Rheumatol. 35: 305–311.
Hollander, A. P., T. F. Heathfield, C. Webber, Y. Iwata, R. Bourne, C. Rorabeck, and A. R. Poole (1994) Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay.J. Clin. Invest. 93: 1722–1732.
Hollander, A. P., I. Pidoux, A. Reiner, C. Rorabeck, R. Bourne, and A. R. Poole (1995) Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration,J. Clin. Invest. 96:2859–2869.
Yang, K. H., J. Parvizi, S.-J. Wang, D. G. Lewallen, R. R. Kinnick, J. F. Greenleaf, and M. E. Bolander (1996) Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture modelJ. Orthop. Res. 14: 802–809.
Zhang, Z.-J., J. Huckle, C. A. Francomano, and R. G. S. Spencer (2002) The influence of pulsed low-intensity ultrasound on matrix production of chondrocytes at different stages of differentiation: an explant study.Ultrasound Med. Biol. 28:1547–1553.
Giannoni, P., A. Crovace, M. Malpeli, E. Maggi, R. Arbico, R. Cancedda, and B. Dozin (2005) Species variability in the differentiation potential ofin vitro-expanded articular chondrocytes restricts predictive studies on cartilage repair using animal models.Tissue Eng. 11: 237–248.
Mott, J. D. and Z. Werb (2004) Regulation of matrix biology by matrix metalloproteinases.Curr. Opin. Cell Biol. 16: 558–564.
Mort, J. S. and C. J. Billington (2001) Articular cartilage and changes in arthritis: Matrix degradation.Arthritis Res. 3: 337–341.
Kafienah, W., F. Al-Fayez, A. P. Hollander, and M. D. Barker (2003) Inhibition of cartilage degradation: a combined tissue engineering and gene therapy approach.Arthritis Rheum. 48: 709–718.
Mix, K. S., M. B. Sporn, C. E. Brinckerhoff, D. Eyre, and D. J. Schurman (2004) Novel inhibitors of matrix metalloproteinase gene expression as potential therapies for arthritis.Clin. Orthop. Relat. Res. 427 Suppl: S129-S137.
Noel, D., D. Gazit, C. Bouquet, F. Apparailly, C. Bony, P. Plence, V. Millet, G. Turgeman, M. Perricaudet, J. Sany, and C. Jorgensen (2004) Short-term BMP-2 expression is sufficient forin vivo osteochondral differentiation of mesenchymal stem cells.Stem Cells 22: 74–85.
Pittenger, M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig, and D. R. Marshak (1999) Multilineage potential of adult human mesenchymal stem cells.Science 284: 143–147.
Johnstone, B., T. M. Hering, A. I. Caplan, V. M. Goldberg, and J. U. Yoo (1998)In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells.Exp. Cell Res. 238: 265–272.
Ma, H. L., S. C. Hung, S. Y. Lin, Y. L. Chen, and W. H. Lo (2003) Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads.J. Biomed. Mater. Res. A 64: 273–281.
Mizuta, H., S. Kudo, E. Nakamura, Y. Otsuka, K. Takagi, and Y. Hiraki (2004) Active proliferation of mesenchymal cells prior to the chondrogenic repair response in rabbit full-thickness defects of articular cartilage.Osteoarthr. Cartil. 12: 586–596.
Indrawattana, N., G. Chen, M. Tadokoro, L. H. Shann, H. Ohgushi, T. Tateishi, J. Tanaka, and A. Bunyaratvej (2004) Growth factor combination for chondrogenic induction from human mesenchymal stem cell.Biochem. Biophys. Res. Commun. 320: 914–919.
Mastrogiacomo, M., R. Cancedda, and R. Quatro (2001) Effect of different growth factors on the chondrogenic potential of human bone marrow stromal cells.Osteoarthr. Cartil. 9 Suppl A: S36-S40.
Angele, P., J. U. Yoo, C. Smith, J. Mansour, K. J. Jepsen, M. Nerlich, and B. Johnstone (2003) Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiatedin vitro.J. Orthop. Res. 21: 451–457.
O'Driscoll, S. W., F. W. Keeley, and R. B. Salter (1988) Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year.J. Bone Joint Surg. Am. 70: 595–606.
Wakitani, S., T. Goto, S. J. Pineda, R. G. Young, J. M. Mansour, A. I. Caplan, and V. M. Goldberg (1994) Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage.J. Bone Joint Surg. Am. 76: 579–592.
Huang, C. Y., K. L. Hagar, L. E. Frost, Y. Sun, and H. S. Cheung (2004) Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells.Stem Cells 22: 313–323.
Ebisawa, K., K. Hata, K. Okada, K. Kimata, M. Ueda, S. Torii, and H. Watanabe (2004) Ultrasound enhances transforming growth factor beta-mediated chondrocyte differentiation of human mesenchymal stem cells.Tissue Eng. 10: 921–929.
Lee, H. J., B. H. Choi, B.-H. Min, Y. S. Son, and S. R. Park (2006) Low-intensity ultrasound stimulation enhances chondrogenic differentiation in alginate culture of mesenchymal stem cells.Artif. Organs 30: 707–715.
De Bari, C., F. Dell'Accio, and F. P. Luyten (2004) Failure ofin vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilagein vivo.Arthritis Rheum. 50: 142–150.
Cui, J. H., S. R. Park, K. Park, B. H. Choi, and B.-H. Min (2007) Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formationin vivo. Tissue Eng. (under revision).
Cui, J. H., K. Park, S. R. Park, and B.-H. Min (2006) Effects of low-intensity ultrasound on chondrogenic differentiation of mesenchymal stem cells embedded in polyglycolic acid: anin vivo study.Tissue Eng. 12: 75–82.
Bai, X., Z. Xiao, Y. Pan, J. Hu, J. Pohl, J. Wen, and L. Li (2004) Cartilage-derived morphogenetic protein-1 promotes the differentiation of mesenchymal stem cells into chondrocytes.Biochem. Biophys. Res. Commun. 325: 453–460.
Schmitt, B., J. Ringe, T. Haupl, M. Notter, R. Manz, G.-R. Burmester, M. Sittinger, and C. Kaps (2003) BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture.Differentiation 71: 567–577.
Bosnakovski, D., M. Mizuno, G. Kim, T. Ishiguro, M. Okumura, T. Iwanaga, T. Kadosawa, and T. Fujinaga (2004) Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system.Exp. Hematol. 32: 502–509.
Park, S.-H., W. Y. Sim, S. W. Park, S. S. Yang, B. H. Choi, S. R. Park, K. Park, and B.-H. Min (2006) An electromagnetic compressive force by cell exciter stimulates chondrogenic differentiation of bone marrow-derived mesenchymal stem cells.Tissue Eng. 12: 3107–3117.
Mankin, H. J. (1982) The response of articular cartilage to mechanical injury.J. Bone Joint Surg. Am. 64: 460–466.
Poole, A. R. (1999) An introduction to the pathophysiology of osteoarthritis.Front. Biosci. 4: 662–670.
Ficat, R. P., C. Ficat, P. Gedeon, and J. B. Toussaint (1979) Spongialization: a new treatment for diseased patellae.Clin. Orthop. Relat Res. 144: 74–83.
Hangody, L., P. Feczko, L. Bartha, G. Bodo, and G. Kish (2001) Mosaicplasty for the treatment of articular defects of the knee and ankle.Clin. Orthop. Relat. Res. 391 Suppl: S328-S336.
Muller, B. and D. Kohn (1999) Indication for and performance of articular cartilage drilling using the Pridie method.Orthopade 28: 4–10.
Brittberg, M., A. Lindahl, A. Nilsson, C. Ohlsson, O. Isaksson, and L. Peterson (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation.N. Engl. J. Med. 331: 889–895.
Peterson, L., T. Minas, M. Brittberg, A. Nilson, E. Sjogren-Jansson, and A. Lindahl (2000) Two-to 9-year outcome after autologous chondrocyte transplantation of the knee.Clin. Orthop. Relat. Res. 374: 212–234.
Hunziker, E. B. (2002) Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects.Osteoarthr. Cartil. 10: 432–463.
Huang, M. H., R. C. Yang, H. J. Ding, and C. Y. Chai (1999) Ultrasound effect on level of stress proteins and arthritic histology in experimental arthritis.Arch. Phys. Med. Rehabil. 80: 551–556.
Park, S. R., S.-H. Park, K. W. Jang, H. S. Cho, J. H. Cui, H. J. An, M. J. Choi, S. I. Chung, and B.-H. Min (2005) The effect of sonication on simulated osteoarthritis. Part II: Alleviation of osteoarthritis pathogenesis by 1 MHz ultrasound with simultaneous hyaluronate injection.Ultrasound Med. Biol. 31: 1559–1566.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Min, BH., Choi, B.H. & Park, S.R. Low intensity ultrasound as a supporter of cartilage regeneration and its engineering. Biotechnol. Bioprocess Eng. 12, 22–31 (2007). https://doi.org/10.1007/BF02931799
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02931799