Abstract
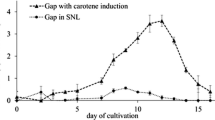
Fluorescein derivatives added into the growth medium were decolorized during submerged cultivation ofPhanerochaete chrysosporium. The highest decrease of absorbanceA 450 was observed in the growth phase regardless of the presence of inducers Tween 80 or poly(ethylene glycol) (PEG). Fluorescein linked to PEG was prepared and, after addition to cultures, shown to stimulate the production of lignin peroxidase. Passing of fluorescing substances into hyphae (observed by confocal microscopy) showed that they were concentrated on some structures inside hyphae.
Similar content being viewed by others
References
Baron-Epel O., Hernandez D., Jiang L.W., Meiners S., Schindler M.: Dynamic continuity of cytoplasmic and membrane compartments between plant cells.J.Cell Biol. 106, 715–721 (1988).
Cole L., Davies D., Hyde G.J., Ashord A.E.: ER-Tracker dye and BODIPY-brefeldin A differentiate the endoplasmatic reticulum and Golgi bodies from the tabular-vacuole system in living hyphae ofPisolithus tinctorius.J.Microsc. 197, 239–248 (2000).
Grgič I., Podgornik H., Berovič M., Perdih A.: Improvements in the determination of manganese peroxidase activity.Biotechnol. Lett. 23, 1039–1042 (2001).
Hammel K.E.: Mechanisms for polycyclic aromatic hydrocarbon degradation by ligninolytic fungi.Environ.Health Perspect. 103, 41–43 (1995).
Jäger A., Croan S., Kirk T.K.: Production of ligninases and degradation of lignin in agitated submerged cultures ofPhanerochaete chrysosporium.Appl.Environ.Microbiol. 50, 1274–1278 (1985).
Katič M., Frantar J., Grgič I., Podgornik H., Perdih A.: Polyoxyethylene stimulates lignin peroxidase production inPhanerochaete chrysosporium.Folia Microbiol. 43, 631–634 (1998).
Kawai S., Jensen K.A., Bao W., Hammel K.E.: New polymeric model substrates for the study of microbial ligninolysis.Appl.Environ.Microbiol. 61, 3407–3414 (1995).
Knapp J.S., Newby P.S., Reece L.P.: Decolorization of dyes by wood-rotting basidiomycete fungi.Enzyme Microb.Technol. 17, 664–668 (1995).
Kos N., Perdih A.: Polyoxirane distribution in aPhanerochaete chrysosporium culture.Folia Microbiol. 44, 527–529 (1999).
Lanz E., Slavík J., Kotyk A.: 2′,7′-Bis-(2-carboxyethyl)-5(6)-carboxyfluorescein as a dual-emission fluorescent indicator of intracellular pH suitable for argon laser confocal microscopy.Folia Microbiol. 44, 429–434 (1999).
Podgornik H., Grgič I., Perdih A.: Decolorization rate of dyes using lignin peroxidases ofPhanerochaete chrysosporium.Chemosphere 38, 1353–1359 (1999).
Podgornik H., Podgornik A., Perdih A.: Kinetic measurements of lignin peroxidase activity.Acta Chim.Slov. 44, 253–260 (1997).
Reddy C.A.: The potential for white-rot fungi in the treatment of pollutants.Curr.Opin.Biotechnol. 6, 320–328 (1995).
Rodriguez Couto S., Dominguez A., Sanroman Á.: Production of managanese-dependent peroxidase in a new solid-state bioreactor byPhanerochaete chrysosporium grown on wood shavings. Application to the decolorization of synthetic dyes.Folia Microbiol. 47, 417–422 (2002).
Rotman B., Papermaster B.W.: Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of florigenic esters.Biochemistry 55, 134–141 (1966).
Sam M., Yesilada O.: Decolorization of Orange II dye by white-rot fungi.Folia Microbiol. 46, 143–146 (2001).
Sani R.K., Azmi W., Banerjee U.C.: Comparison of static and shake culture in the decolorization of textile dyes and dye effluents byPhanerochaete chrysosporium.Folia Microbiol. 43, 85–88 (1998).
Stewart A., Deacon J.W.: Vital fluorochromes as tracers for fungal growth studies.Biotech.Histochem. 70, 57–65 (1995).
Swamy J., Ramsay J.A.: The evaluation of white rot fungi in the decolorization of textile dyes.Enzyme Microb.Technol. 24, 130–137 (1999).
Tsuji T., Kawasaki Y., Takeshima S., Sekiya T., Tanaka S.: A new fluorescence staining assay for visualizing living microorganisms in soil.Appl.Environ.Microbiol. 61, 3415–3421 (1995).
Verma P., Madamwar D.: Decolorization of synthetic textile dyes by lignin peroxidase ofPhanerochaete chrysosporium.Folia Microbiol. 47, 283–286 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grgič, I., Perdih, A. Passing of fluorescein derivatives into the hyphae ofPhanerochaete chrysosporium . Folia Microbiol 48, 199–202 (2003). https://doi.org/10.1007/BF02930956
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02930956