Abstract
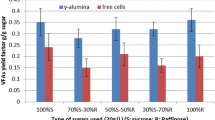
Using γ-alumina pellets, more than threefold increase of the ethanol productivity in the fermentation molasse has been obtained in the present work. Also, molasse fermentation in the presence of γ-alumina gave 78.4 g/L ethanol, ethanol yield factor 0.44 g/g, and conversion 89.4% at initial sugar concentration (ISC) 179.5 g/L, compared to 53.9 g/L, 0.30 g/g and 62.7% in its absence, respectively. Furthermore, it was found that γ-alumina reduces the activation energy Ea of fermentation. This inorganic material does not promote the fermentation of raisin extract.
Similar content being viewed by others
References
Koutinas, A. A., Kanellaki, M., Lycourghiotis, A., Typas, M. A., and Drainas, C. (1988),J. Appl. Microbiol. Biotechnol. 28, 235–239.
Kanellaki, M., Koutinas, A. A., Kana, K., Nikolopoulou, M., Papadimitriou, A., and Lycourghiotis, A. (1989),Biotechnol. Bioeng. 34(1), 121–125.
Koutinas, A. A. and Kanellaki, M. (1990),J. Food Sci. 55(2), 525–531.
Kana, K., Kanellaki, M., Papadimitriou, A., Psarianos, C., and Koutinas, A. A. (1989),J. Ferment. Bioeng. 68(3), 213–215.
Tsoutsas, T., Kanellaki, M., Psarianos, C., Kalliafas, A., and Koutinas, A. A. (1990),J. Ferment. Bioeng. 69(2), 93–97.
Swings, J. and Deley, J. (1977),Bacteriol. Rev. 41, 1–46.
Daugulis, A. J., Brown, N. M., Cluett, W.R., and Dunlop, D. B. (1981),Biotechnol. Lett. 3(11), 651–656.
Margaritis, A. and Rowe, C. E. (1983),Dev. Ind. Microbiol. 24, 329–336.
Margaritis, A., Bajpai, P., and Wallace, J. (1981),Biotechnol. Lett. 3, 613–618.
Rouxhet, P. G., Van Haecht, J. L., Didelez, J., Gerard, P., and Briquet, M. (1981),Enzym. Microb. Technol. 3, 49–54.
Rogers, P. L., Lee, K. J, Scotnicki, M. L., and Triebe, D. E. (1982),Adv. Biochem. Eng. Biotechnol. 23, 37–84.
Koutinas, A. A., Kanellaki, M., Typas, M. A., and Drainas, C. (1986),Biotechnol. Lett. 8(7), 517–520.
Scott, T. A. and Melvin, E. H. (1953),Anal. Chem. 25, 1651–1661.
Giannakoudakis, D., ed. (1964),Chemical Kinetic, University of Thessaloniki, Greece, p. 110.
Author information
Authors and Affiliations
Additional information
Author to whom all corresnondence and reprint requests should be addressed.
Rights and permissions
About this article
Cite this article
Iconomou, L., Psarianos, C., Kanellaki, M. et al. The promotion of molasse alcoholic fermentation usingSaccharomyces cerevisiae in the presence of γ-alumina. Appl Biochem Biotechnol 31, 83–96 (1991). https://doi.org/10.1007/BF02922128
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02922128