Abstract
The studies to improve the production of glutaryl-7-ACA from cephalosporin C are described in this paper.
During the conversion of cephalosporin C to keto-adipyl-7-aminocephalosporonic acid by d-amino acid oxidase (d-AAO), with the simultaneous production of equimolar amount of hydrogen peroxide, an incomplete nonenzymatic conversion of the keto form into the glutaryl form occurs, where cephalosporin C as well asd-AAO are partly destroyed in the presence of hydrogen peroxide.
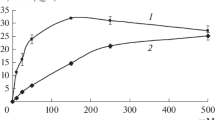
d-AAO was immobilized to different carriers in order to achieve better enzyme stability. The activity of immobilizedd-AAO on manganese oxide remained above 100% during the first 9 h of a semicontinuous conversion of cephalosporin C. The presence of catalase coimmobilized with D-AAO and coupled to CNBr-activated Sepharose 4B improved the operation stability ofd-AAO.
An additional approach for the continuous transformation of cephalosporin C used whole cells ofTrigonopsis variabilis, containingd-AAO, immobilized to magnetic iron oxide particles.
Similar content being viewed by others
Abbreviations
- PMSF:
-
phenylmethylsulfonyl fluoride
- 2,4-DNPH:
-
2,4-dinitrophenylhydrazine
- D-AAO:
-
D-amino acid oxidase
- PEG:
-
polyethylene glycol
- CPG:
-
controlled pore glass
References
Fildes, R. A., Potts, J. R., and Farthing, J. E. (1974). United States Patent 3,801,458.
Bergström, J., Fürst, P., Josephson, B., and Noree, L.-O. (1972),Nutr. Metabol. 14, Suppl., pp. 162–170.
Brodelius, P., Nilsson, K., and Mosbach, K. (1981),Appl. Biochem. Biotechnol. 6, 293.
Szwajcer, E., Brodelius, P., and Mosbach, K. (1982),Enzyme Microb. Technol. 4, 409.
Wikström, P., Szwajcer, E., Brodelius, P., Nilsson, K., and Mosbach, K. (1982),Biotechnol. Lett. 4, 153.
Kubicek-Pranz, E. M., and Röhr, M., (1985),Biotechnol. Lett. 7, 9.
Deshphande, A., Sanicaran, K., D’Souza, S. F., and Nadkarani, G. B. (1987),Biotechnol. Techniques 1, 55.
Szwajcer, E. and Mosbach, K. (1985),Biotechnol. Lett. 7, 1.
Cuatrecasas, P., Wilchek, M., and Anfinsen, C. B. (1968),Proc. Natl. Acad. Sci. USA 61, 636.
Nilsson, K. and Mosbach, K. (1981),Biochem. Biophys. Res. Commun. 102, 449.
Miller, A. W. and Robyt, J. F. (1983),Biotechnol. Bioeng. 25, 2795.
Duvnjak, Z. and Lilly, M. D. (1976),Biotechnol. Bioeng. 18, 737.
Szwajcer Dey, E., Miller, J., Kovacevic, S., and Mosbach, K. (1990), accepted for publication in “Biochemistry International.”
Alberti, B. N. and Klibanov, A. M. (1982),Enzyme Microb. Technol. 4, 47.
Aldercreutz, P. and Mattiasson, B. (1984),Enzyme Microb. Technol. 20, 296.
Flygare, S. and Larsson, P.-O. (1987),Enzyme Microb. Technol. 9, 494.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dey, E.S., Flygare, S. & Mosbach, K. Stabilization ofd-amino acid oxidase from yeastTrigonopsis variabilis used for production of glutaryl-7-aminocephalosporanic acid from cephalosporin C. Appl Biochem Biotechnol 27, 239–250 (1991). https://doi.org/10.1007/BF02921538
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02921538