Abstract
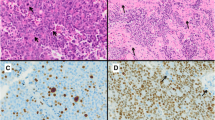
To assess the proliferative activity of pituitary adenomas, 36 surgically removed adenomas were studied by light microscopical parameters; mitotic count; expression of PCNA, Ki-67, cathepsin D, and EGF; and image cytometry. Three adenomas (9%) showed high, 11 (34%) medium, 17 (53%) moderate, and 1 (3%) low structural differentiation. In 10 adenomas (31%), no mitosis was observed. The average was 2.4 mitoses/100 HPF; the highest count was 7.1 mitoses/100 HPF. Eleven adenomas (33.3%) were PCNA-negative; in 20 adenomas (60.6%), between 0.05 and 3.9, and in 2 adenomas (6.0%), between 10.5 and 16.4 PCNA-positive nuclei were observed. Only a recurrent null-cell adenoma (9%) was Ki-67-negative. Three adenomas (9.1%) were EGF-negative, 28 (84.8%) showed up to 10% positive cells, and 2 (6.1 %) showed between 10 and 30% positive cells; 19 adenomas (68%) were cathepsin D-negative, including all endocrine-inactive adenomas. Half the adenomas had an euploid DMA stem line. Endocrine-inactive adenomas displayed a higher rate of euploid DNA stem lines than endocrine-active adenomas. The S-phase fraction varied between 2.97 and 28%, with a mean value of 14.4%. Half the adenomas showed an S-phase fraction of 11.65% or lower.
Similar content being viewed by others
References
Ahyai A, Hori A, Bockermann V, Rama B, Blech M, Markakis E. Impulse cytophotometric DNA analysis in pituitary adenomas. Neurosurg Rev 11:77–86, 1988.
Anniko M, Holm LE, Stilverswärd C, Tribukait B, Wersäll J. Cellular DNA content of pituitary tumours: Its relationship to morphology and hormonal type of tumour. Acta Otolaryngol 379 (suppl):13–19, 1981.
Anniko M, Holm LE, Tribukait B, Werner S, Wersäll J. The clinical implications of DNA-characteristics in human pituitary adenomas. Acta Otolaryngol 379 (suppl): 21–28, 1981.
Anniko M, Tribukait B, Wersäll J. DNA ploidy and cell phase in human pituitary tumors. Cancer 53:1708–1713, 1984.
Anniko M, Wersäll J. DNA studies for prediction of prognosis of pituitary adenomas? In: Landolt AM, Heitz PU, Zapf J, Girard J, Del Pozo E, eds. Advances in pituitary adenoma research, vol 69. New York: Pergamon Press, 1988. pp. 45–52.
Barlogie B, Drewinko B, Schuhmann J, Göhde W, Dosik G, Johnston DA, Freireich EJ. Cellular DNA content as a marker of neoplasia in man. Am J Med 69:195–203, 1980.
Bravo R, Fey SJ. Identification of a nuclear and of a cytoplasmic polypeptide whose relative proportions are sensitive to changes in the rate of cell proliferation. Exp Cell Res 136:311–319, 1981.
Bravo R, Frank R. Cyclin/PCNA is the auxiliary protein of DNA polymerase delta. Nature 326:515–517, 1987.
Bravo R, McDonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: Association with DNA replication sites. J Cell Biol 105:1549–1554, 1987.
Brown DC, Gatter KC. Monoclonal antibody Ki-67: Its use in histopathology. Histopathology 17:489–503, 1990.
Carpenter G, Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol 88:227–237, 1975.
Carpenter G, Wahl MI. Peptide growth factors and their receptors. In: Born GVR, ed. Handbook experimental pharmacology, vol 95. Berlin, Heidelberg: Springer-Verlag, 1990, pp. 1–168.
Cattoretti G, Becker MH, Key G, Duchrow M, Schlüter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (Mib 1 and 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 168:357–363, 1992.
Celis JE, Celis A. Cell cycle dependent variation in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: Subdivision of S-phase. Proc Natl Acad Sci USA 82:3262–3266, 1985.
Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the newborn animal. J Biol Chem 237:1555–1562, 1962.
Dervan PA, Magee HM, Carney DN. Proliferating cell nuclear antigen counts in formalin-fixed paraffin-embedded tissue correlate with Ki-67 in fresh tissue. Am J Clin Pathol 97:S21–28, 1992.
Duffy MJ, Brouillet JP, Reilly D, McDermott E, O’Higgins N, Fennelly JJ, Maudelonde T, Rochefort H. Cathepsin D concentration in breast cancer cytosols: Correlation with biochemical, histological, and clinical findings. Clin Chem 37:101–104, 1991.
Ezzat S, Melmed S. The role of growth factors in the pituitary. J Endocr Invest 13:691–698, 1990.
Feulgen R, Rossenbeck H. Mikroskopischchemischer Nachweis einer Nucleinsäure vom Typus der Thymonucleinsäure. Hoppe-Seyler’s Zeitschrift für physiologische Chemie 135:202–248, 1923.
Fitzgibbons PL, Appley AJ, Turner RR, Bishop PC, Parker JW, Breeze RE, Weiss MH, Apuzzo MLJ. Flow cytometric analysis of pituitary tumors. Correlation of nuclear antigen p105 and DNA content with clinical behavior. Cancer 62:1556–1560, 1988.
Friedlander ML, Hedley DW. Clinical and biological significance of aneuploidy in human tumours. J. Clin Pathol 37:961–974, 1984.
Fu YS, Hall TL. DNA ploidy measurements in tissue sections. Anal Quant Cytol Histol 7:90–96, 1985.
Garcia M, Lacombe MJ, Duplay H, Cavailles V, Derocq D, Delarue JC, Krebs B, Contesso G, Sancho-Garnier H, Richer G, Domergue J, Namer M, Rochefort H. Immunohistochemical distribution of the 52-kDa protein in mammary tumors: A marker associated with cell proliferation rather than with hormone responsiveness. J Steroid Biochem Mol Biol 27:429–445, 1987.
Gerdes J, Lemke H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133:1710–1715, 1984.
Gerdes J. An immunohistological method for estimating cell growth fractions in rapid histopathological diagnosis during surgery. Int J Cancer 35:169–171, 1985.
Gerdes J, Becker MHG, Key G, Cattoretti G. Immunohistochemical detection of tumour growth factor (Ki-67 antigen) in formalin-fixed and routinely processed tissue. J Pathol 168:85–86, 1992.
Gilchrist CA, Shull JD. Epidermal growth factor induces prolactin mRNA in GH4C1 cells via a protein synthesis-dependent pathway. Mol Cell Endocrinol 92:201–206, 1993.
Hall PA, Levison DA, Woods AL, Yu CCW, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R, Waseem NH, Lane DP. Proliferating cell nuclear antigen (PCNA) immunologicalisation in paraffin sections. An index of cell proliferation with deregulated expression in some neoplasm. J Pathol 162:285–290, 1990.
Hall PA, Levison DA, Wright NA. Assessment of cell proliferation in clinical practise. Heidelberg: Springer-Verlag, 1992.
Hall TL, Fu YS. Biology of disease: Applications of quantitative microscopy in tumor pathology. Lab Invest 53:5–21, 1985.
Hauguel-DeMouzon S, Csermely P, Zoppini G, Kahn CR. Quantitative dissociation between EGF effects on c-myc and c-fos gene expression, DNA synthesis, and epidermal growth factor receptor tyrosinase activity. J Cell Physiol 150:180–187, 1992.
Henry JA, McCarthy AL, Angus B, Westley BR, May FEB, Nicholson S, Cairns J, Harris AL, Hörne CHW. Prognostic significance of the estrogen-regulated protein, Cathepsin D, in breast cancer. An immunohistochemical study. Cancer 65:265–271, 1990.
Hiddemann W, Schumann J, Andreeff M, Barlogie B, Herman CJ. Convention on nomenclature for DNA cytometry. Cytometry 5:445–446, 1984.
Hsu DW, Hakim F, Biller BMK, De LaMonteS, Zervas NT, Klibanski A, Hedley-Whyte ET. Significance of proliferating cell nuclear antigen index in predicting pituitary adenoma recurrence. J Neurosurg 78:753–761, 1993.
Hsu SM, Raine L, Fänger H. The use of antiavidin antibody and avidin-biotin-per- oxidase complex in immunoperoxidase technics. Am J Clin Pathol 75:816–821, 1981.
Hulting AL, Tribukait B, Bergstrand G, Werner S. DNA and S-phase representation in human growth hormone producing pituitary adenomas. Acta Endocrinol (Copenh) 109:295–303, 1985.
Joensuu H, Klemi PJ. DNA aneuploidy in adenomas of the endocrine organs. Am J Pathol 132:145–151, 1988.
Kasselberg AG, Orth DN. Immunocytochemical localisation of human epidermal growth factor/urogastrone in several human tissues. J Histochem Cytochem 33: 315–322, 1985.
Kurki P, Vanderlaan M. Expression of proliferating cell nuclear antigen (PCNA)/ cyclin. Exp Cell Res 166:209–219, 1986.
Landolt AM, Shibata T, Kleihues P, Tuncdogan E. Growth of human pituitary adenomas: Facts and speculations. In: Landolt AM, Heitz PU, Zapf J, Girard J, Del PozoE, eds. Advances in pituitary adenoma research, vol 69. New York: Pergamon Press, 1988. pp 53–62.
Lüdecke DK, Beck-Bornholdt HP, Saeger W, Schmidt W. Tumour ploidy in DNA histograms of pituitary adenomas. Acta Neurochir 76:18–22, 1985.
Mathews MB, Bernstein RM. Identity of the proliferating cell nuclear antigen and cyclin. Nature 309:374–376, 1984.
Mellin W. Cytophotometry in tumour pathology. Pathol Res Pract 186:37–62, 1990.
Miyachi K, Fritzler MJ. Autoantibody to a nuclear antigen in proliferating cells. J Immunol 121: 2228–2234, 1978.
Moolenar WH, Bierman AJ, Tilly BC, Verlann I, Defize LHK, Honegger AM, Ullrich A, Schlessinger J. A point mutation at the ATP binding site of the EGF receptor abolishes signal transduction. EMBO J 7: 707–710, 1988.
Montcourrier P, Mangeat PH, Salazar G, Morisset M, Sahuquet A, Rochefort H. Cathepsin D in breast cancer cells can digest extracellular matrix in large acidic vesicles. Cancer Res 50:6045–6054, 1990.
Nagashima T, Murovic JA, Hoshino T, et al. The proliferative potential of human pituitary tumors in situ. Neurosurg 64:588–593, 1986.
Reid WA, Valler MJ, Kay J. Immunolocalisation of Cathepsin D in normal and neoplastic human tissue. J Clin Pathol 39: 1323–1330, 1986.
Renner U, Mojto J, Arzt E, Lange M, Stalla J, Müller OA, Stalla G. Secretion of polypeptide growth factors by human nonfunctioning pituitary adenoma cells in culture. Neuroendocrinology 57:825–834, 1993.
Rosa JC, Mendes R, Filipe MI, Morris RW. Measurement of cell proliferation in gastric carcinoma: Comparative analysis of Ki-67 and proliferative cell nuclear antigen (PCNA). Histochem J 24:93–101, 1992.
Sachs L. Angewandte Statistik. New York: Springer-Verlag, 1992.
Saeger W. Die Hypophysentumoren. Cytologische und ultrastrukturelle Klassifikation, Pathogenese, endokrine Funktionen und Tierexperiment. In: Büngeler W, Eder M, Lennert K, Peters G, Sandritter W, Scifert G, eds. Veröffentlichungen aus der Pathologie, Band 107. Stuttgart: G. Fischer, 1977. Pp 1–240.
Saeger W. Simmonds Memorial Lecture: Pituitary adenoma classification. Endocr Pathol 3:S42-S45, 1992.
Sandritter W. Allgemeine Pathologic New York: FK Schattauer, 1981.
Schlessinger J. Epidermal growth factor receptor as a multifunctional allosteric protein. Biochemistry 27:3119–3123, 1988.
Silvestrini R, Costa A, Veneroni S, DelBinoG, Persici P. Comparative analysis of different approaches to investigate cell kinetics. Cell Tissue Kinet 21:123–131, 1988.
Start RD, Cross SS, Clelland C, Silcocks PB, Rogers K, Smith JHF. Delay in fixation does not affect the immunoreactivity of proliferating cell nuclear antigen (PCNA). J Pathol 168:197–199, 1992.
Stoscheck CM, King LE. Role of epidermal growth factor in carcinogenesis. Cancer Res 46:1030–1037, 1986.
Tan CK, Castillo C, SO AG, Downey KM. An auxiliary protein for DNA polymerase delta from fetal calf thymus. Biol Chem Hoppe Seyler 261:12310–12316, 1986.
Tandon AK, Clark M, Chamness GC, Chirgwin JM, McGuire WL. Cathepsin D and prognosis in breast cancer. N Engl J Med 322:297–302, 1990.
Verheijen R, Kuippers HJH. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. J Cell Sci 92:123–130, 1989.
Waseen NH, Lane DP. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci 96:121–129, 1990.
Zbieranowski I, Murray D. The study of endocrine tumors by flow and image cytometry. Endocr Pathol 3:63–82, 1992.
Zhang K, Kulig E, Jin L, Lloyd RV. Effects of estrogen and epidermal growth factor on prolactin and Pit-1 mRNA in GH3 cells. Proc Soc Exp Biol Med 202:193–200, 1993.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krämer, A., Saeger, W., Tallen, G. et al. DNA measurement, proliferation markers, and other factors in pituitary adenomas. Endocr Pathol 5, 198–211 (1994). https://doi.org/10.1007/BF02921487
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02921487