Abstract
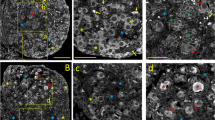
In addition to their well-known involvement in neuromuscular junctions and in brain cholinergic synapses, cholinergic mechanisms have been implicated in the growth and maturation of oocytes in various species. Functional acetylcholine receptors were electrophysiologically demonstrated in amphibian and mammalian oocyte membranes, and activity of the acetylcholine-hydrolyzing enzyme, acetylcholinesterase (AChE), was biochemically measured in the exceptionally big oocytes of the frogXenopus laevis. However, biochemical methods could not reveal whether AChE was produced within the oocytes themselves or in the surrounding follicle cells. Furthermore, this issue is particularly important for understanding growth and fertilization processes in the much smaller human oocytes, in which the sensitivity of AChE biochemical measurements is far too low to be employed. To resolve this question, a molecular biology approach was combined with biochemical measurements on ovarian extracts and sections. To directly determine whether the human cholinesterase (ChE) genes are transcriptionally active in oocytes, and, if so, at what stages in their development, the presence of ChE mRNA was pursued. For this purpose frozen ovarian sections were subjected to in situ hybridization using35S-labeled human ChE cDNA. Highly pronounced hybridization signals were localized within oocytes in primordial, preantral, and antral follicles, but not in other ovarian cell types, demonstrating that within the human ovary ChE mRNA is selectively synthesized in viable oocytes at different developmental stages. Sucrose gradient centrifugation followed by [3H]acetylcholine hydrolysis measurements revealed in the ovarian extracts the presence of low levels of soluble AChE dimers, sensitive to the specific AChE inhibitor BW284C51 but resistant to the BuChE inhibitor iso-OMPA. In view of the low numbers of oocytes out of total cells in the ovary, these findings strongly suggest that AChE is a prominent protein in human oocytes throughout their development and further support the hypothesis that cholinergic mechanisms may be involved in oocyte growth and maturation in humans.
Similar content being viewed by others
References
Austin, L., Berry, W.K. (1953). Two selective inhibitors of cholinesterase. Biochem J. 54:695–700
Branks, P.L., Wilson, M.C. (1986). Patterns of gene expression in the murine brain revealed by in situ hybridization of brain-specific mRNAs. Mol. Brain Res. 1:1–6
Caratsch, C., Eusebi, F., Salustri, A. (1984). Acetylcholine receptors in monkey and rabbit oocytes. J. Cell. Physiol. 121:415–418
Cox, K.H., DeLeon, D.V., Angerer, L.M., Angerer, R.C. (1984). Detection of mRNAs in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev. Biol. 101:485–502
Dascal, N., Landau, E.M. (1980). Types of muscarinic response inXenopus oocytes. Life Sci. 27:1423–1428
Dascal, N., Yekuel, R., Oron, Y. (1984). Acetylcholine promotes progesterone-induced maturation inXenopus oocytes. J. Exp. Zool. 230:131–135
Drews, U. (1985). Cholinesterase in embryonic development. Prog. Histochem. Cytochem. 7:1–52
Egozi, Y., Sokolovsky, M., Schejter, E., Blatt, I., Zakut, H., Matzkel, A., Soreq, H. (1986). Divergent regulation of muscarinic binding sites and of acetylcholines-terase in discrete regions of the developing human fetal brain. Cell. Mol. Neurobiol. 6:66–70
Eppig, J.J., Downs, S.M. (1984). Chemical signals that regulate mammalian oocyte maturation. Biol. Reprod. 30:1–11
Eusebi, F., Mangia, F., Alfei, I. (1979). Acetylcholine-elicted responses in primary and secondary mammalian oocytes disappear after fertilization. Nature 277:651–653
Eusebi, F., Pasetto, N., Siracusa, G. (1984). Acetylcholine receptors in human oocytes. J. Physiol. 346:321–330
Flormann, H.M., Storey, B.T. (1981). Inhibition of in vitro fertilization of mouse eggs: 3-Guinciclidinyl benzilate specifically blocks penetration of zonae pellucidae by mouse spermatozoa. J. Exp. Zool. 216:159–167
Gundersen, C.B., Miledi, R. (1985). Acetylcholinesterase activity ofXenopus Laevis oocytes. Neuroscience 10:1487–1495
Johnson, C.D., Russell, R.L. (1975). A rapid, simple radiometric assay for cholinesterase, suitable for multiple determinations. Anal. Biochem. 64:229–238
Kusano, K., Miledi, R., Stinnakre, J. (1977). Acetylcholine receptors in the oocyte membrane. Nature 270:739–741
Layer, P.G. (1983). Comparative localization of acetylcholinesterase and pseudocholinesterase during morphogenesis of the chicken brain. Proc. Natl. Acad. Sci. U.S.A. 80:413–417
Layer, P.G., Sporns, O. (1987). Spatiotemporal relationship of embryonic cholinesterases with cell proliferation in chicken brain and eye. Proc. Natl. Acad. Sci. U.S.A. 84:284–288
Levanon, D., Lieman-Hurwitz, J., Dafni, N., Wigderson, M., Sherman, L., Bernstein, Y., Laver-Rudich, Z., Danciger, E., Stein, O., Groner, Y. (1985). Architecture and anatomy of the chromosomal locus in human chromosome 21 encoding the CuZn-superoxide dismutase. EMBO J. 4:77–84
Lockridge, O., LaDu, B. (1986). Amino acid sequence of the active site of human serum ChE from usual, atypical and atypical-silent genotypes. Biochem. Genetics 24:485–498
Maniatis, T., Fritsch, E.F., Sambrook, J. (1982). Molecular Cloning and Laboratory Mannual. Cold Spring Harbor Laboratory. Cold Spring Harbor, New York
Massoulie, J., Bon, S. (1982). The molecular forms of ChE and AchE in vertebrates. Annu. Rev. Neurosci. 5:57–106
Oron, Y., Dascal, N., Nadler, E., Lupu, M. (1985). Inositol 1,4,5-triphosphate mimics muscarinic response inXenopus oocytes. Nature 313:141–143
Owman, Ch., Sjoberg, N.O., Svensson, K.G., Walles, B. (1975). Autonomic nerves mediating contractility in the human graafian follicle. J. Reprod. Fertil 45:553–566
Picard, A., Giraud, F., Le Bouffant, F., Sladeczek, F., Le Pench, C., and Doree, M. (1985). Inositol 1,4,5-triphosphate microinjection triggers activation, but not meiotic maturation in amphibian and starfish oocytes. FEBS Lett. 182:446–450
Prody, L., Zevin-Sonkin, D., Gnatt, A., Koch, R., Zisling, R., Goldberg, O., Soreq, H. (1986). Use of synthetic oligodeoxynucleotide probes for the isolation of a human ChEcDNA clone. J. Neurosci. Res. 16:25–35
Prody, C., Zevin-Sonkin, D., Gnatt, A., Goldberg, O., Soreq, H. (1987). Isolation and characterization of full length cDNA clones coding for cholinesterase from fetal human tissues. Proc. Natl. Acad. Sci. U.S.A. 84:3555–3559
Razon, N., Soreq, H., Roth, E., Bartal, A., Silman, I. (1984). Characterization of levels and forms of cholinesterases in human primary brain tumors. Exp. Neurol. 84:681–695
Sastry, B.V.R., Sadavongvivad, C. (1979). Cholinergic systems in non-nervous tissues. Pharmacol. Rev. 39:65–132
Soreq, H. (1985). The biosynthesis of biologically active proteins in mRNA-microinjectedXenopus oocytes: CRC Crit. Rev. Biochem. 18:199–238
Soreq, H., Gnatt, A. (1987). Molecular biological search for human genes encoding cholinesterases. Mol. Neurobiol. 1:47–80
Soreq, H., Miskin, R. (1984). Secreted proteins in the medium and microinjectedXenopus oocytes are degraded by oocyte proteases. FEBS Lett. 128:305–310
Soreq, H., Parvari, R., Silman, I. (1982). Biosynthesis and secretion of catalytically active acetylcholinesterase inXenopus oocytes microinjected with mRNA from rat brain and from torpedo electric organ. Proc. Natl. Acad. Sci. U.S.A. 79:830–835
Soreq, H., Zevin-Sonkin, D., Razon, N. (1984). Expression of cholinesterase gene(s) in human brain tissue: Translational evidence for multiple mRNA species. EMBO J. 3:1371–1375
Soreq, H., Zevin-Sonkin, D., Avni, A., Hall, L.M.C., Spierer, P. (1985). A human acetylcholinesterase gene identified by homology to theDrosophila gene. Proc. Natl. Acad. Sci. U.S.A. 82:1827–1831
Southern, E.M. (1975). Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503–517
Woodland, H.R., Wilt, F.H. (1980). The functional stability of sea urchin histone mRNA injected into oocytes ofXenopus laevis. Dev. Biol. 75:199–204
Wramsby, H., Fredga, K., Liedholm, P. (1987). Chromosome analysis of human oocytes recovered from preovulatory follicles in stimulated cycles. N. Engl. J. Med. 316:121–124
Zakut, H., Matzkel, A., Schejter, E., Avni, A., Soreq, H. (1985). Polymorphism of acetylcholinesterase in discrete regions of the developing human fetal brain. J. Neurochem. 45:382–389
Author information
Authors and Affiliations
Additional information
This research was supported by the Medical Research and Development Command of the U.S. Army (contract DAMD-17-87-C7169, to H.S.), by the Research Fund at the Edith Wolfson Medical Center (to H.Z.) by the Israel Health Ministry (to H.S. and H.Z.), and by the Israel Association for Cancer Research (to G.M.)
Rights and permissions
About this article
Cite this article
Malinger, G., Zakut, H. & Soreq, H. Cholinoceptive properties of human primordial, preantral, and antral oocytes: In situ hybridization and biochemical evidence for expression of cholinesterase genes. J Mol Neurosci 1, 77–84 (1989). https://doi.org/10.1007/BF02918893
Issue Date:
DOI: https://doi.org/10.1007/BF02918893