Abstract
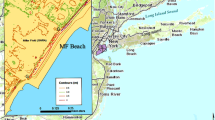
Four populations of the native annual grassTriplasis purpurea were surveyed on coastal beaches along the south shore of Staten Island, NY, to determine the potential of this species to colonize shoreline habitats mostly devoid of other vegetation. If the species can establish and maintain dense populations, it may have conservation value for urban beaches disturbed by human activities. For two populations, survivorship, growth, and reproduction were monitored at different distances from shore to determine the ability of this species to maintain viable populations. At three sites,T. purpurea occurred in >75% of all quadrats and the highest density was 1195 plants/m2 at 74 m from shore in one recently disturbed site. Density generally increased with increasing distances from shore at low tide (from ca. 40 – 90 m). Plants showed the greatest growth and reproduction at close distances to shore (30 – 40 m); part of this effect was due to density in one population, but when density effects were removed statistically, there still remained a decline in growth and reproduction with increasing distance from shore. Improved vigor nearest to shore may be due to continual sand deposition. Survivorship showed a Type I pattern, with low mortality throughout the growing season. By colonizing newly-deposited and continually shifting sands,T. purpurea can contribute to the earliest stages of ecological succession along disturbed beaches in eastern North America and may be valuable to the development and management of urban coastal plant communities.
Similar content being viewed by others
References
Barbour, M.G., de Jong, T.M. & Pavlik, B.M. 1985. Marine beach and dune plant communities. In: Chabot, B.F. & Mooney, H.A. (eds.)Physiological ecology of North American plant communities, pp. 296–322. Chapman & Hall, New York, NY.
Bazzaz, F.A. & Morse, S.R. 1991. Annual plants: potential responses to multiple stresses. In: Mooney, H.A., Winner, W.E. & Pell, E.J. (eds.)Responses of plants to multiple stresses, pp. 283–305. Academic Press, New York, NY.
Bertness, M.D. 1999.The ecology of Atlantic shorelines. Sinauer Associates, Inc., Sunderland, MA.
Bonanno, S.E., Leopold, D.J. & Hilaire, L.R. 1998. Vegetation of a freshwater dune barrier under high and low recreational uses.J. Torrey Bot. Soc. 125: 40–50.
Bonham, C.D. 1989.Measurements for terrestrial vegetation. John Wiley & Sons, New York, NY.
Boyce, S.G. 1954. The salt spray community.Ecol. Monogr. 24: 29–67.
Bullock, J. 1996. Plants. In: Sutherland, W.J. (ed.)Ecological census techniques: a handbook, pp. 111–138. Cambridge University Press, Cambridge.
Burden, R.F. & Randerson, P.F. 1972. Quantitative studies of the effects of human trampling on vegetation as an aid to the management of semi-natural areas.J. Appl. Ecol. 9: 439–457.
Carlson, L.H. & Godfrey, P.J. 1989. Human impact management in a coastal recreation and natural area.Biol. Conserv. 49: 141–156.
Castillo, S.A. & Moreno-Casasola, P. 1996. Coastal sand dune vegetation: an extreme case of species invasion.J. Coastal Conserv. 2: 13–22.
Chase, A. 1918. Axillary cleistogenes in some American grasses.Am. J. Bot. 5: 254–258.
Cheplick, G.P. 1996a. Cleistogamy and seed heteromorphism inTriplasis purpurea (Poaceae).Bull. Torrey Bot. Club 123: 25–33.
Cheplick, G.P. 1996b. Do seed germination patterns in cleistogamous annual grasses reduce the risk of sibling competition?J. Ecol. 84: 247–255.
Cheplick, G.P. 1998. Seed dispersal and seedling establishment in grass populations. In: Cheplick, G.P. (ed.)Population biology of grasses, pp. 84–105. Cambridge University Press, Cambridge.
Cheplick, G.P. & Demetri, H. 1999. Impact of saltwater spray and sand deposition on the coastal annualTriplasis purpurea (Poaceae).Am. J. Bot. 86: 703–710.
Cheplick, G.P. & Grandstaff, K. 1997. Effects of sand burial on purple sandgrass (Triplasis purpurea): the significance of seed heteromorphism.Plant Ecol. 133: 79–89.
Cheplick, G.P. & Wickstrom, V.M. 1999. Assessing the potential for competition on a coastal beach and the significance of variable seed mass inTriplasis purpurea.J. Torrey Bot. Soc. 126: 296–306.
Cook, R.P. & Tanacredi, J.T. 1990. Management strategies for increasing habitat and species diversity in an urban national park. In:Ecosystem management: rare species and significant habitats, pp. 248–250. New York State Museum Bull. 471.
Davis, J.H. 1956. Influences of man upon coast lines. In: Thomas, W.L., Sauer, C.O., Bates, M. & Mumford, L. (eds.)Man’s role in changing the face of the earth, pp. 504–521. University of Chicago Press, Chicago, IL.
Day, R.W. & Quinn, G.P. 1989. Comparisons of treatments after an analysis of variance in ecology.Ecol. Monogr. 59: 433–463.
Deevey, E.S. 1947. Life tables for natural populations of animals.Quart. Rev. Biol. 22: 283–314.
Dowdy, S. & Wearden, S. 1991.Statistics for research. John Wiley & Sons, New York, NY.
Duncan, W.H. & Duncan, M.B. 1987.The Smithsonian guide to seaside plants of the Gulf and Atlantic coasts from Louisiana to Massachusetts, exclusive of lower Peninsular Florida. Smithsonian Institution Press, Washington, DC.
Eriksson, E. & Ehrlen, J. 1992. Seed and microsite limitation of recruitment in plant populations.Oecologia (Berl.) 91: 360–364.
Fox, G.A. 1993. Failure-time analysis: emergence, flowering, survivorship, and other waiting times. In: Scheiner, S.M. & Gurevitch, J. (eds.)Design and analysis of ecological experiments, pp. 253–289. Chapman & Hall, New York, NY.
Hesp, P.A. 1991. Ecological processes and plant adaptations on coastal dunes.J. Arid. Environ. 21: 165–191.
Hitchcock, A.S. 1950.Manual of the grasses of the United States, 2nd ed. (revised by A. Chase). United States Department of Agriculture Miscellaneous Publication 200, Washington, DC.
Hosier, P.E. & Eaton, T.E. 1980. The impact of vehicles on dune and grassland vegetation on a southeastern North Carolina barrier beach.J. Appl. Ecol. 17: 173–182.
Jefferies, R.L., Davy, A.J. & Rudmik, T. 1981. Population biology of the salt marsh annualSalicornia europaea agg.J. Ecol. 69: 17–31.
Keddy, P.A. 1981. Experimental demography of the sand-dune annual,Cakile edentula, growing along an environmental gradient in Nova Scotia.J. Ecol. 69: 615–630.
Liddle, M.J. & Greig-Smith, P. 1975. A survey of tracks and paths in a sand dune ecosystem. II. Vegetation.J. Appl. Ecol. 12: 909–930.
Mack, R.N. 1976. Survivorship ofCerastium atrovirens at Aberffraw, Anglesey.J. Ecol. 64: 309–312.
Mack, R.N. & Pyke, D.A. 1983. The demography ofBromus tectorum: variation in space and time.J. Ecol. 71: 69–93.
Maun, M.A. 1994. Adaptations enhancing survival and establishment of seedlings on coastal dune systems.Vegetatio 111: 59–70.
Maun, M.A. 1998. Adaptations of plants to burial in coastal sand dunes.Can. J. Bot. 76: 713–738.
McDonnell, M.J. 1981. Trampling effects on coastal dune vegetation in the Parker River National Wildlife Refuge, Massachusetts, USA.Biol. Conserv. 21: 289–301.
Muenchow, G. 1986. Ecological use of failure time analysis.Ecology 67: 246–250.
Oosting, H.J. & Billings, W.D. 1942. Factors effecting [sic] vegetational zonation on coastal dunes.Ecology 23: 131–142.
Ozturk, M. 1999. Urban ecology and land degradation. In: Farina, A. (ed.)Perspectives in ecology, pp. 115–120. Backhuys Publishers, Leiden.
Pyke, D.A. & Thompson, J.N. 1986. Statistical analysis of survival and removal rate experiments.Ecology 67: 240–245.
Sokal, R.R. & Rohlf, F.J. 1981.Biometry. W.H. Freeman, San Francisco, CA.
Stalter, R. 1993. Dry coastal ecosystems of the eastern United States of America. In: van der Maarel, E. (ed.)Dry Coastal Ecosystems: Africa, America, Asia and Oceania, pp. 317–340. Elsevier, Amsterdam.
Stalter, R., Byer, M.D. & Tanacredi, J.T. 1996. Rare and endangered plants at Gateway National Recreation Area: a case for protection of urban natural areas.Landscape Urban Plan. 35: 41–51.
Symonides, E. 1988. Population dynamics of annual plants. In: Davy, A.J., Hutchings, M.J. & Watkinson, A.R. (eds.)Plant population ecology, pp. 221–248. Blackwell Scientific, Oxford.
Tanacredi, J.T. 1983. Coastal zone management practices at an urban national park.Environ. Manage. 7: 143–150.
Tanacredi, J.T. 1987. Natural resource management policy constraints and trade-offs in an urban national recreation area. In: Adams, L.W. & Leedy, D.L. (eds.)Integrating man and nature in the metropolitan environment, pp. 221–227. National Institute for Urban Wildlife, Columbia, MD.
Underwood, A.J. 1997.Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge.
van der Valk, A.G. 1974. Environmental factors controlling the distribution of forbs on coastal foredunes in Cape Hatteras National Seashore.Can. J. Bot. 52: 1057–1073.
Watkinson, A.R. 1990. The population dynamics ofVulpia fasciculata: a nine-year study.J. Ecol. 78: 196–209.
Watkinson, A.R. & Davy, A.J. 1985. Population biology of salt marsh and sand dune annuals.Vegetatio 62: 487–497.
Watkinson, A.R. & Harper, J.L. 1978. The demography of a sand dune annual:Vulpia fasciculata. I. The natural regulation of populations.J. Ecol. 66: 15–33.
Watkinson, A.R., Huiskes, A.H.L. & Noble, J.C. 1979. The demography of sand dune species with contrasting life cycles. In: Jefferies, R.L. & Davy, A.J. (eds.)Ecological processes in coastal environments, pp. 95–112. Blackwell Scientific, Oxford.
Woodhouse, W.W. Jr. 1982. Coastal sand dunes of the U.S. In: Lewis, R.R. III (ed.)Creation and restoration of coastal plant communities, pp. 1–44. CRC Press, Boca Raton, FL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheplick, G.P., Demetri, H. Population biology of the annual grassTriplasis purpurea in relation to distance from shore on Staten Island, New York. J Coast Conserv 6, 145–154 (2000). https://doi.org/10.1007/BF02913811
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02913811