Abstract
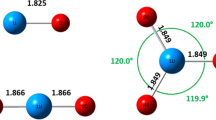
The molecular structure of ThF4 is studied by synchrotron electron diffractometry and mass spectrometry. The thermally mean values of internuclear distances are found at a temperature of 1370±20 K: rg(Th-F) = 2.124(5) å, rg(F-F) = 3.418(31) å, δ(F-F) = 0.049(32) å, and LF-Th-F = 107.2(1.7)‡. These distances agree with the tetrahedral structure of the molecule. The missing vibration frequencies of the ThF4 molecule are calculated in a combined analysis of electron diffraction and spectroscopic data: v1 = 620(30) cm-1 and v2 = 115(15) cm-1.
Similar content being viewed by others
References
N. I. Giricheva, O. G. Krasnova, and G. V. Girichev,Zh. Strukt. Khim.,39, No. 2, 239–246 (1998).
R. J. M. Konings, A. S. Booji, A. Kovacs, et al.,J. Mol. Struct.,378 121–131 (1996).
A. Buchler, J. B. Berkowitz-Mattuck, and D. H. Dugre,J. Chem. Phys.,34, No. 6, 2202–2206 (1961).
V. N. Bukhmarina, A. Yu. Gerasimov, Yu. B. Predtechenskii, and V. G. Shklyarik,Opt. Spektrosk.,72, No. 1, 69–74 (1992).
V. N. Bukhmarina, Yu. B. Predtechenskii, and V. G. Shklyarik,ibid.,62, No. 5, 1187–1188 (1987).
Yu. S. Ezhov, P. A. Akishin, and N. G. Rambidi,Zh. Strukt. Khim.,10, No. 4, 571–575 (1969).
K. H. Lau, R. D. Brittain, and D. L. Hildenbrand,J. Chem. Phys.,90, No. 2, 1158–1164 (1989).
G. V. Girichev, S. A. Shlykov, and Yu. F. Revichev,Prib. Tekh. éksp., No. 4, 167–169 (1986).
N. I. Giricheva, E. Z. Zasorin, and G. V. Girichev,Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol.,17, No. 4, 616–618 (1974).
V. P. Mikulin,Photographic Compounding Handbook [in Russian], Iskusstvo, Moscow (1972).
L. I. Ermolaeva, E. Z. Zasorin, and B. S. Butaev, “Software for structural analysis of molecules by gas electron diffractometry. 1. Program for primary processing of experimental data,” Moscow State University (1979), VINITI dep. No. 4203 (1979).
R. A. Bonham and L. Schafer,International Tables of X-Ray Crystallography, Birmingham (1973).
B. Andersen, H. M. Seip, T. G. Strand, and R. Stolevic,Acta Chem. Scand.,21, 3224–3234 (1969).
L. E. Alexander and I. R. Beattie,J. Chem. Soc, Dalton Trans.,16, 1745–1750 (1972).
V. N. Bukhmarina, S. L. Dobychin, Yu. B. Predtechenskii, and V. G. Shklyarik,Zh. Phys. Khim.,60, No. 7, 1775–1777 (1986).
V. P. Spiridonov, A. G. Gershikov, E. Z. Zasorin, and B. S. Butaev,Diffraction Studies on Noncrystalline Substances, Akademiai Kiado, Budapest (1981), pp. 159–195.
R. H. Hauge, J. W. Hasti, and J. L. Margrave,J. Less-Common Metals,23, 359 (1971).
J. T. Waber and D. T. Cromer,J. Chem. Phys.,42, 4116–4123 (1965).
A. S. Dworkin,J. Inorg. Nucl. Chem.,34, 135–138 (1972).
D. L. Hildenbrand,J. Chem. Phys.,66, 4788 (1977).
Author information
Authors and Affiliations
Additional information
Translated fromZhurnal Strukturnoi Khimii, Vol. 40, No. 2, pp. 251–258, March–April, 1999.
Rights and permissions
About this article
Cite this article
Girichev, G.V., Krasnov, A.V., Giricheva, N.I. et al. Molecular structure and vibrational characteristics of thorium tetrafluoride. J Struct Chem 40, 207–213 (1999). https://doi.org/10.1007/BF02903648
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02903648