Abstract
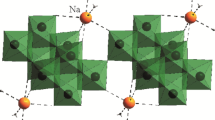
Crystal structures of Cs4[Re6Te8(CN)6]·2H2O (1) and Ba2[Re6Te8(CN)6]· 12H2O (2) are determined. Crystals 1 are orthorhombic, a = 14,282(1), b = 12.910(1), c = 18.040(1) Å, Vcell = 3326.3(8) Å3, space group Pbcn, Z = 4, dcalc = 5.715 g/cm3, R(F) = 0.0482 for 3193 Fhkl > 4σ(F). Crystals 2 are triclinic, a = 9.671(3), b = 9.697(4), c = 11.039(4) Å, α = 89.86(3), β = 72.34(3), γ = 82.46(3)∘, Vcell = 977.2(6) Å3, space group P1, Z = 1, dcalc = 4.733 g/cm3, R(F) = 0.0490 for 3226 Fhkl > 4σ(F). In both structures, the [Re6Te8(CN)6]4− anions form a distorted primitive cubic packing with distances between the centers 9.02-9.63 Å in 1 and 9.70-11.04 Å in 2. The Cs+ cations in 1 lie near the face centers of the cubes formed by the onions. In 2, cation pairs (Ba2+)2 bonded to two solvate water molecules are formed; the pairs lie at the centers of the anion cubes. In structures 1 and 2, there are shortened contacts between the tellurium atoms belonging to the neighboring anions (3.75-4.09 and 3.95-4.22 Å, respectively).
Similar content being viewed by others
References
Yu. V. Mironov, A. V. Virovets, V. E. Fedorov, et al.,Polyhedron,14, 3171–3173 (1995).
A. Slougui, Yu. V. Mironov, A. Perrin, and V. E. Fedorov,Croat. Chem. Acta,68, 885–890 (1995).
Yu. V. Mironov, J. A. Cody, T. E. Albrecht-Schmitt, and J. A. Ibers,J. Am. Chem. Soc.,119, No. 3, 493–498 (1996).
V. E. Fedorov, S. V. Tkachev, N. G. Naumov, et al., to appear in:Zh. Neorg. Khim.
G. M. Sheldrick,Acta Crystallogr.,A46, 467–473 (1990).
G. M. Sheldrick,ibid.,A49 (Suppl.), C53 (1993).
N. G. Naumov, A. V. Virovets, N. V. Podberezskaya, and V. E. Fedorov,Zh. Strukt. Khim.,38, No. 5, 1018–1024 (1997).
F. Klaiber, W. Petter, and F. Hulliger,J. Solid State Chem.,46, No. 1, 112–120 (1983).
V. E. Fedorov, N. V. Podberezskaya, A. V. Mishchenko, et al.,Mater. Res. Bull.,21, No. 11, 1335–1342 (1986).
V. P. Fedin, V. E. Fedorov, H. Imoto, and T. Saito,Polyhedron,16, 1615–1619 (1997).
Yu. V. Mironov, M. Pell, and J. A. Ibers,Angew. Chem. Int. Ed. Engl,35, Nos. 23/24, 2854–2856 (1996).
S. S. Batsanov,Zh. Neorg. Khim.,36, No. 12, 3015–3047 (1991).
Author information
Authors and Affiliations
Additional information
Translated fromZhurnal Struktumoi Khimii, Vol. 39, No. 5, pp. 885–893, 1998, September–October, 1998.
Rights and permissions
About this article
Cite this article
Imoto, H., Naumov, N.G., Virovets, A.V. et al. Primitive cubic packing of anions in Cs4[Re6Te8(CN)6]· 2H2O and Ba2[Re6Te8(CN)6] · 12H2O crystals. J Struct Chem 39, 720–727 (1998). https://doi.org/10.1007/BF02903545
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02903545