Abstract
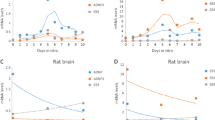
RNA was extracted from five different rat brain regions during development, starting from embryonic day 15 (E15) until postnatal day 60 (P60). These RNA preparations were analyzed by both Northern and dot blot for their content of 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase), myelin proteolipid protein (PLP), and myelin basic protein (MBP) -specific transcripts. CNPase mRNA was readily detectable at E15 and PLP mRNA at P1 in all brain regions examined. In contrast, expression of MBP mRNA followed a caudorostral gradient. It was first observed at P1 in the mesencephalon and at P9-P11 in the olfactory bulb. Expression of these three transcripts displayed two types of developmental profiles. One was termed biphasic because the specific mRNA level increased regularly and then reached a plateau level. The other developmental profile was termed triphasic, because there was a gradual increase in the level of specific transcripts with a sudden appearance of a sharp peak followed by a decline to a plateau level. When the triphasic pattern was observed, the date of the peak appearance was probe-, but not region-, dependent. It was P15 for CNPase, P18 for MBP, and P21 for PLP. As these peaks occurred at a time during development when myelination was the most active, we postulate the existence of a transient external signal, perhaps neuronal, which would be responsible for this increased amount of myelin-related transcripts.
Similar content being viewed by others
References
Anderson, M.L.M., Young, B.D. (1985) Quantitative filter hybridisation. Nucleic Acid Hybridisation, A Practical Approach B.D. Hames, S.J. Higgins (eds). IRL Press, Oxford, pp 73–113
Aviv, H., Leder, P. (1972) Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc. Natl. Acad. Sci. U.S.A. 69:1408–1412
Bains, W., Ponte, P., Blau, H., Kedes, L. (1984) Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Mol. Cell. Biol. 4:1449–1453
Bernier, L., Alvarez, F., Norgard, E.M., Raible, D.W., Mentaberry, A., Shemri, J.G., Sabatini, D.D., Colman, D.R. (1987) Molecular cloning of a 2′,3′-cyclic nucleotide 3′-phosphohydrolase: mRNAs with different 5′ ends encode the same set of proteins in nervous and lymphoid tissues. J. Neurosci. 7:2703–2710
Campagnoni, A.T., Hunkeler, M.J. (1980) Synthesis of the myelin proteolipid protein in the developing mouse brain. J. Neurobiol. 11:355–364
Chirgwin, J.M., Przybyla, A.E., MacDonald, R.J., Rutter, W.J. (1979) Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18:5294–5299
Civelli, O., Birnberg, N, Herbert, E. (1982) Detection and quantification of pro-opiomelanocortine mRNA in pituitary and brain tissues from different species. J. Biol. Chem. 255:6782–6792
Dautigny, A., Alliel, P.M., d’Auriol, L., Pham Dinh, D., Nussbaum, J.L., Gallibert, F., Jolles, P. (1985) Molecular cloning and nucleotide sequence of a cDNA coding for the rat brain myelin proteolipid. FEBS Lett. 188:33–36
Delassalle, A., Zalc, B., Lachapelle, F., Raoul, M., Collier, P., Jacque, C. Regional distribution of myelin basic protein in the CNS of quaking, jimpy and normal mice during development and aging. J Neurosci. Res. 6:303–313
Dubois-Dalcq, M., Behar, T., Hudson, L., Lazzarini, R.A. (1986) Emergence of three myelin proteins in oligodendrocytes cultured without neurons. J. Cell Biol. 102:384–392
Faucon-Biguet N., Buda M., Lamouroux A., Samolyk D., Mallet J. (1986) Time course of the changes of TH mRNA in rat brain and adrenal medulla after single injection of reserpine. EMBO J. 5:287–291
Gardinier, M.V., Macklin, W.B., Diniack, A.J., Deininger, P.L. (1986) Characterization of myelin proteolipid mRNAs in normal and Jimpy mice. Mol. Cell. Biol. 6:3755–3762
Levi G., Gallo V., Ciotti M.T. (1986) Bipotential precursors of putative fibrous astrocytes and oligodendrocytes in rat cerebellar cultures express distinct surface features and “neuron-like” gamma-aminobutyric acid transport. Proc. Natl. Acad. Sci. U.S.A. 83:1504–1508
Macklin, W.B., Weill, C.L., Deininger, P.L. (1986) Expression of myelin proteolipid and basic protein mRNAs in cultured cells. J. Neurosci. Res. 16:203–217
Mikoshiba, K., Nagaike, K., Tsukada, Y. (1980) Subcellular distribution and developmental change of 2′,3′-cyclic nucleotide 3′-phosphohydrolase in the central nervous system of the myelin-deficientshiverer mutant mice. J. Neurochem. 35:465–470
Milner, R.J., Lai, C., Nave, K.A., Lenoir, D., Ogata, J., Sutcliffe, J.G. (1985) Nucleotide sequences of two mRNA for rat brain myelin proteolipid protein. Cell 42:931–939
Monge, M., Kadiisky, D., Jacque, C., Zalc, B. (1986) Oligodendroglial expression and deposition of four major myelin constituents in the myelin sheath during development: An in vivo study. Dev. Neurosci. 8:222–235
Monge, M., Zalc, B., Kanfer, J. (1988) Regional and developmental estimations of UDP galactose:ceramide galactosyl transferase activity in the rat brain. Dev. Neurosci. 10:43–46
Naismith, A.L., Hoffman-Chudzik, E., Tsui, L.-C., Riordan, J.R. (1985) Study of the expression of myelin proteolipid protein (lipophilin) using a cloned complementary DNA. Nucleic Acids Res. 13:7413–7425
Paterson, J.A., Privat, A., Ling, E.A., Leblond, C.P. (1973) Investigation of glial cells in semithin sections III. Transformation of subependymal cells into glial cells, as shown by radioautography after3H thymidine injection into the lateral ventricle of the brain of young rats. J. Comp. Neurol. 149:83–102
Privat, A., Leblond, C.P. (1972) The subependymal layer and neighboring region in the brain of the young rat. J. Comp. Neurol. 146:277–302
Raff, M.C., Miller, R.H., Noble, M. (1983) A glial progenitor cell that develops in vitro into in an astrocyte or an oligodendrocyte depending on culture medium. Nature 303:390–396
Raff, M.C., Abney, E.R., Fok-Seang, J. (1985) Reconstitution of a developmental clock in vitro: A critical role for astrocytes in the timing of oligoden-drocyte differentiation. Cell 42:61–69
Rigby, P.W.J., Dieckman, M., Rhodes, C., Berg, P. (1977) Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J. Mol. Biol. 113:237–251
Roach, A., Boylan, K., Horvath, S., Prusiner, S.B., Hood, L.E. (1983) Characterization of cloned cDNA representing rat myelin basic protein: absence of expression in brain of shivere mutant mice. Cell 34:799–806
Roth, H.J., Kronquist, K., Pretorius, P.J., Crandall, B.F., Campagnoni, A.T. (1986) Isolation and characterization of a cDNA coding for a novel human 17.3K myelin basic protein (MBP) variant. J. Neurosci. Res. 16:227–238
Sprinkle, T.J., Zaruba, M.E., McKhann, G.M. (1978) Activity of 2′,3′-cyclic-nucleotide 3′-phosphodiesterase in regions of rat brain during development: Quantitative relationship to myelin basic protein. J. Neurochem. 30:309–314
Thomas P. (1980) Hybridization of denaturated RNA and DNA fragments transferred to nitrocellulose. Proc. Natl. Acad. Sci. U.S.A. 77:5201–5205
Weydert, A., Barton, P., Harris, A.J., Pinset, C., Buckingham, M. (1987) Developmental pattern of mouse skeletal myosin heavy chain gene transcripts in vivo and in vitro. Cell 49:121–129
Yakovlev, P.I., Lecours, A.R. (1967) The myelogenetic cycles of regional maturation of the brain. Regional Developments of the Brain in Early Life. Minkowski (ed). Blackwell Scientific, Oxford, pp 3–71
Zeller, N.K., Hunkeler, M.J., Campagnoni, A.T., Sprague, J.A., Lazzarini, R.A. (1984) Characterization of mouse myelin basic protein messenger RNAs with a myelin basic protein cDNA clone. Proc. Natl. Acad. Sci. U.S.A. 81:18–22
Author information
Authors and Affiliations
Additional information
This work was supported by an Association de Recherche pour la Sclérose en Plaques grant (to B.Z.) and a Canadian Medical Council grant (to J.K.).
Rights and permissions
About this article
Cite this article
Kanfer, J., Parenty, M., Goujet-Zaıc, C. et al. Developmental expression of myelin proteolipid, basic protein, and 2′,3′-cyclic nucleotide 3′-phosphodiesterase transcripts in different rat brain regions. J Mol Neurosci 1, 39–46 (1989). https://doi.org/10.1007/BF02896855
Issue Date:
DOI: https://doi.org/10.1007/BF02896855