Abstract
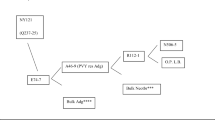
Potato varieties of Europe, widely grown prior to the late blight epidemic of the 1840s, were apparently derived mainly from ChileanSolanum tuberosum Group (Gp) Tuberosum and with contributions from Gp Andigena. A small number of these old varieties had field resistance and consequently survived the late blight. These survivors, along with a limited number of 19th and early 20th century introductions, provided the very narrow genetic base for our modern potato variety development. Beginning in the first half of the 20th century, resistance to diseases and pests from exotic species and primitive relatives was backcrossed into the existing parental stocks, with little improvement in broadening of the genebase. By the 1980s, 77% of European and somewhat fewer North American varieties had genes, derived by backcrossing, fromS. demissum (late blight resistance) and Andigena (resistance to cyst nematode). Broadening of the Tuberosum genebase was undertaken in 1959 by creating long-day adapted Neo-Tuberosum (N-T) from large populations of Andigena. This took six or more cycles of recurrent mass selection. Simmonds, in England, was the first to begin this work, followed shortly after by Plaisted, in the U.S., and Tarn, in Canada. Varieties with N-T in their pedigrees include the New York releases “Rosa”, which is 50% N-T, and “Eva”, 25% N-T. The Tuberosum genebase has also been broadened with diploid Gp Phureja resulting in the releases of “Yukon Gold,”with yellow flesh and high internal quality, and “NorValley,”a chipper with resistance to cold sweetening. Over 5000 accessions of about 150 wild species are available to breeders from the U.S. Department of Agriculture, National Research Support Project 6 (NRSP-6) genebank. Many of these accessions have been evaluated for resistance to diseases and pests as well as other important traits. Six genebanks in other countries also have many accessions for breeders. These seven collections are a great source of valuable traits for breeding, but remain under-utilized, mainly because of the time and additional resources required in eliminating the “wildness”characters associated with the desired traits. “Pre-breeding”is needed to help breeders utilize the many needed genes and alleles in the wild species. There now are two projects with pre-breeding as an objective in the U.S., one at Madison, WI, and the other at Prosser, WA. Resistance to cold sweetening (low sugar build up in cold storage) has been backcrossed from several wild species into the Tuberosum background, as has resistance to late blight, the Columbia rootknot nematode, and the potato leafroll virus (PLRV). Resistance to potato virus Y (PVY) and PLRV obtained from N-T has been incorporated into Tuberosum parental stocks. Durable resistance to late blight in Polish breeding stocks, withS. demissum andS. stoloniferum background, and in improved Bolivian and Peruvian Andigena has also been utilized by North American programs.
Resumen
Las variedades de papa de Europa, cuyo crecimiento se difundió antes de la epidemia del tizón tardío de 1840, en apariencia se derivaron principalmente de la papa chilena Solanum tuberosum grupo (Gp) Tuberosum con contribución del Gp Andigena. Un pequeño número de esas var-iedades antiguas tenía resistencia de campo y, en consecuencia, logró sobrevivir a la epidemia. Esas sobrevivientes, conjuntamente con un número limitado de introducciones realizado durante los siglos 19 y 20, proporcionaron la muy estrecha base genética para nuestro moderno desarrollo de variedades de papa. En la primera mitad del siglo 20 se comenzaron los retrocruzamientos para resistencia a enfermedades y plagas desde las especies exóticas y sus parientes primitivos al stock existente de progenitores, con pequeños progresos en el ensanchamiento de la base genética. Para 1980, el 77 por ciento de las papas europeas y unas pocas de Norteamérica tenían genes, derivados de retrocruzamientos, de S. demissum (con resistencia al tizón tardío) y Andigena (resistencia al nematodo del quiste). El ensanchamiento de la base genética de Tuberosum se emprendió en 1959 con la creación de una Neo-Tuberosum (N-T) adaptada a dias largos, a partir de grandes poblaciones de Andigena. Este proceso tomó seis o más ciclos de selección masiva recurrente. Simmonds, en Inglaterra, fue el primero en iniciar dichos trabajos, seguido de cerca por Plaisted, en los Estados Unidos, y Tarn en Canadá. Las variedades con N-T en sus pedigrees incluyen la liberatión de “Rose” en Nueva York, la cual contiene 50% de N-T, y Eva, con 25% de N-T. La base genética de Tuberosum también ha sido ampliada con el diploide del Gp Phureja, lo cual ha resultado en la liberación de “Yukon Gold”, con pulpa amarilla y gran calidad interna, y “Nor Valley”, para hojuelas, con resistencia al endulzamiento en frío. Alrededor de 4300 accesiones de más de 100 especies silvestres están disponibles para los mejoradores en el banco genético del Proyecto 6 Apoyo a los Investigadores Nacionales (NRSP-6) del Departamento de Agricultura de los Estados Unidos. Muchas de esas accesiones han sido evaluadadas por su resistencia a enfermedades y plagas así como por otras importantes características. Seis bancos genéticos en otros países también tienen muchas accesiones disponibles para los mejoradores. Estas siete colecciones son una gran fuente de invalorables características para fitomejoramiento, pero permanecen subutilizadas, principalmente debido al tiempo y los recursos adicionales requeridos para eliminar las caracteristicas de “rusticidad” asociadas con los caracteres deseados. El “premejo-ramiento” es necesario para ayudar a los fitomejoradores a utilizar los múltiples genes y alelos más útiles de las especies silvestres. En los Estados Unidos existai actualmente dos proyectos cuyo objetivo es el premejoramiento, uno está en Madison, WI, y el otro en Prosser, WA. La resistencia al endulzamiento en frío (formatión de poco azúcar durante al almacenamiento en frío) ha sido retro-cruzada a la base de Tuberosum a partir de diversas especies silvestres, según tengan resistencia al tizón tardío, al nematodo del nódulo de la raíz de Columbia o al virus del enrollamiento de la hoja de papa (PLRV). Las resistencia al virus Y de la papa (PVY) y a PLRV obtenida de N-T ha sido incorporada al sotck de progenies de Tuberosum. Los programas de Norteamérica también están usando resistencia duradera al tizón tardío en los stocks de selectión de Polish, con base en S. demissum y S. stoloniferum, y a una Andigena mejorada boliviana y peruana.
Similar content being viewed by others
Literature Cited
Akeley, R.V., W.R. Mills, C.E. Cunningham, and J. Watts. 1968. Lenape: A new potato variety high in solids and chipping quality. Am Potato J 45:142–145.
Austin, S., E. Lojkowska, M.K. Ehlenfeldt, A Kelman, and J.P. Helgeson. 1988. Fertile interspecific somatic hybrids ofSolanum: A novel source of resistance toErwinia soft rot. Phytopathology 78:1216–1220.
Austin, S., J.D. Pohlman, C.R. Brown, H. Mojtahedi, G.S. Santo, D.S. Douches, and J.P. Helgeson. 1993. Interspecific somatic hybridization betweenSolanum tuberosum L. andS. buibocastanum Dun. as a means of transferring nematode resistance. Am Potato J 70:485–495.
Bamberg, J.B., CA. Longtine, and E.B. Radcliffe. 1996a. Fine screeningSolanum potato germplasm accessions for resistance to Colorado Potato Beetle. Am Potato J 73:211–223.
Bamberg, J.B., M.W. Martin, and J.J. Schartner. 1994. Elite Selections of Tuber-bearingSolanum species (based on evaluations for disease, pest, and stress resistance). Potato Introduction Station, NRSP-6, Sturgeon Bay, WI, USA. 56 p.
Bamberg, J.B., M.W. Martin, J.J. Schartner, and D.M. Spooner. 1996b. Inventory of Tuber-bearingSolanum Species. Catalog of Potato Germplasm. Potato Introduction Station, NRSP-6, Sturgeon Bay, WI. USA 110 p.
Brown, C. R., D. Corsini, J. Pavek, and P. Thomas. 1997. Heritability of field resistance to potato leafroll virus in cultivated tetraploid potato. Plant Breeding 116:585–588.
Brown, C.R., M. McNabay, and B.B. Dean. 1999a Genetic characterization of reduced melanin formation in tuber tissueof Solanum hjertingii and hybrids with cultivated diploids. Am J Potato Res 76:37–43.
Brown, C.R., H. Mojtahedi, and G.S. Santo. 1991. Resistance to Columbia root-knot nematode inSolanum ssp. and in hybrids of S.hougasii with tetraploid cultivated potato. Am Potato J 69:445–452.
Brown, C.R., H. Mojtahedi, and G.S. Santo. 1993. Resistance toMeloidogyne chitwoodi derived fromS. bulbocastanum. Am Potato J 70:799. Abstr.
Brown, C.R., H. Mojtahedi, and G.S. Santo. 1999b. Genetic analysis of resistance toMeloidogyne chitwoodi introgressed fromSolanum hougasii in cultivated potato. J Nematology 31:264–271.
Brown, C.R. and P.E. Thomas. 1994. Resistance to potato leafroll virus derived fromSolanum chacoense: characterization and inheritance. Euphytica 74:51–57.
Brown, C.R., C.-P.Yang, H. Mojtahedi, G.S. Santo, and R. Masuelli. 1996. RFLP analysis of resistance to Columbia root-knot nematode derived fromSolanum bulbocastanum in a BC2 population. Theor Appl Genet 92:572–576.
Bukasov, S.M. 1978. Systematics of the potato.In: Kameraz, A.Ya. (ed), Systematics, breeding, and Seed Production of Potatoes. 1985 English Translation. Oxonian Press, New Dehli. pp. 1–42.
Burbank, L. 1914. Luther Burbank: His methods and discoveries and their practical application. Luther Burbank Press, Vol. VII, Chapter EX. New York & London.
Chase, R.W. 1992. American potato variety inventory. Potato Association of America. Certification Section. 12 p.
Colon, L. 1994. Resistance toPhytophthora infestans inSolanumuberosum and wildSolanum species. Thesis, Wageningen, NL. 159 p. CIP Gegevens Koninklyke Bibliotheek, Den Haag.
Corsini, D., J. Pavek, C. Brown, D. Inglis, M. Martin, M. Powelson, A. Dorrance, and H. Lozoya-Saldana. 1999. Late blight resistant potato germplasm release: AWN86514-2. Am J Potato Res 76:45–49.
Dale, F.B. and M.M.de Scurrah. 1998. Breeding for resistance to the potato cyst nematodesGlobodera rostochiensis andGloboderu pallida: strategies, mechanisms and genetic resources.In: Marks, R.J., and B.B. Brodie (eds), Potato Cyst Nnematodes: Biology, Distribution, and Control. CAB International, Wallingford. pp. 177–195.
De Jong, H. 1981. Inheritance of russeting in cultivated diploid potatoes. Potato Res 24:309–313.
De Jong, H., and G.C.C. Tai. 1977. Analysis of tetraploid-diploid hybrids in cultivated potatoes. Potato Res 20:111–121.
De Jong, H., G.C.C. Tai, W.A. Russell, G.R. Johnston, and K.G. Proudfoot. 1981. Yield potential and genotype-environment interactions of tetraploid-diploid (4x-2x) potato hybrids. Am Potato J 58:191–199.
De Jong, H. and T.R. Tarn. 1984. Using germplasm in potato breeding in Canada. Canada Agriculture 30:12–14.
Glendinning, D.R. 1975. Neo-Tuberosum: new potato breeding material. 1. The origin, composition, and development of the Tuberosum. and Neo-Tuberosum gene pools. Potato Res 18:256–261.
Glendinning, D.R. 1976. Neo-Tuberosum: new potato breeding material. 4. The breeding system of Neo-Tuberosum, and the structure and composition of the Neo-Tuberosum gene-pool. Potato Res 19:27–36.
Glendinning, D.R. 1983. Potato introductions and breeding up to the early 20th century. New Phytol 94:479–505.
Goth, R.W., KG. Haynes, and D.R. Wilson. 1994a. Verticillium wilt resistant germplasm: release of russet clone B0169-56. Am Potato J 71:735–742.
Goth, R.W., KG. Haynes, and D.R. Wilson. 1994b. Independent segregation in potato for resistance to Verticillium wilt and pink-eye. Plant Disease 78:562–564.
Goth, R.W., and KG. Haynes. 1996. The germplasm release of B0718-3 and B0767-2: Two late blight resistant potato clones. Am Potato J 74:337–345.
Grun, P. 1974. Cytoplasmic sterilities that separate the Group Tuberosum cultivated potato from its putative tetraploid ancestor. Evolution 27:633–643.
Grun, P., C. Ochoa, and D. Capage. 1977. Evolution of cytoplasmic factors in tetraploid cultivated potatoes (Solanaceae). Am J Bot 64:412–420.
Hamernik, A.J., and R.E. Hanneman Jr. 1998. Breeding 2x haploid x species potatoes that chip from 2 C cold storage. Am Potato J 75:279. Abstr.
Hanneman Jr., R.E. 1989. The potato germplasm resource. Am Potato J 66:655–667.
Hanneman Jr., R.E. 1999. Techniques to transfer germplasm from 2x (1EBN) Mexican species to 2x (2EBN) material via hybridization. Am Potato J 76:371. Abstr.
Hawkes, J.G. 1956. Taxonomic studies on the tuber-bearing Solanums. I.Solanum tuberosum and the tetraploid complex. Proc. Linn. Soc. 166:97–144.
Hawkes, J.G. 1979. Genetic poverty of the potato in Europe. Proc. Conf. Broadening Genet. Base Crops, Wageningen, 1978. Pudoc, Wageningen, pp. 19-27.
Hawkes, J.G. 1990. The Potato: Evolution, Biodiversity, and Genetic Resources. Smithsonian Institution Press. Washington, DC. 259 p.
Haynes, KG. 2000. Inheritance of yellow-flesh intensity in diploid potatoes. J Amer Soc Hort Sci 125: 63–65.
Haynes, KG., R.W. Goth, and R.J. Young. 1997. Genotype x environment interactions for resistance to common scab in tetraploid potato. Crop Sci 37:1163–1167.
Haynes, K G., D. R. Wilson and M. S. Kang. 1995. Genotype x environment interaction for specific gravity in diploid potatoes. Crop Science 35:977–981.
Helgeson, J.P., J.D. Pohlman, S. Austin, G.T. Haberlach, S.M. Wielgus, D. Ronis, L. Zambolim, P.Tooley, J.M. McGrath, R.V. James, and W.R. Stevenson. 1998. Somatic hybrids betweenSolanum bulbocastanum and potato: a new source of resistance to late blight. Theor Appl Gen 96:738–742.
Hermsen, J.G.Th. 1989. Current use of potato collections.In: Brown, A.H.D., O.H. Frankel, D.R. Marshall, and J.T. Williams (eds), The Use of Plant Genetic Resources. Cambridge University Press, Melbourne, pp. 68–87.
Hosaka, K 1986. Who it the mother of the potato?-Restriction endonuclease analysis of chloroplast DNA of cultivated potatoes. Theor Appl Gen 72:606–618.
Hosaka, K 1995. Successive domestication and evolution of the Andean potatoes as revealed by chloroplast DNA restriction endonuclease analysis. Theor Appl Gen 90:356–363.
Hosaka,K, and R.E. Hanneman Jr. 1988. The origin of the cultivated tetraploid potato based on chloroplast DNA. Theor Appl Gen 76:172–176.
Hougas, R.W., and R.W. Ross. 1956. The use of foreign introductions in breeding American potato varieties. Am Potato J 33:328–339.
Huamán, Z. 1998. Collection, maintenance, and evaluation of potato genetic resources. Plant Varieties& Seeds 11:29–38.
Huamán, Z., A. Golmirzaie, and W. Amoros. 1997. The Potato.In: Fuccillo, D., L. Sears, and P. Stapleton (s), Biodiversity in Trust: Conservation and Use of Plant Genetic Resources in CGIAR Centres. Cambridge University Press, Cambridge, pp. 21–28.
Huamán, Z., R. Hoekstra, and J.B. Bamberg. 2000. The inter-genebank potato database and the dimensions of available wild potato germplasm. Am J Potato Res 77:353–362.
Jackson, M.T.,J.G. Hawkes, and P.R. Rowe. 1980. An enthnobotanical field study of primitive potato varieties in Peru. Euphytica 29:328–339.
Jansky, S. 2000. Breeding for disease resistance in potato. Plant Breeding Reviews 19:69–155.
Johnston, G.R., and R. G. Rowberry. 1981. Yukon Gold: A new yellow- fleshed, medium early, high quality table and french-fry cultivar. Am Potato J 58: 241–244.
Johnston, G.R., R.G. Rowberry, and J.F. Alex. 1983. Conestoga: A new early potato cultivar with very good table and chipping qualities. Am Potato J 60:193–197.
Kawchuk, L.M., D.R. Lynch, R.R. Martin, G.C. Kozub, and B. Farries. 1997. Field resistance to the potato leafroll luteovirus in transgenic and somaclone potato plants reduces tuber disease symptoms. Can J Plant Pathology 19:260–266.
Kuhl, J.C., R.E.Hanneman Jr., and M.J. Havey. 1998. Investigation of resistance toPhytophtkora infestans in 2x(lEBN) wild potato species. Am Potato J 75:284. Abstr.
Love, S.L. 1999. Founding clones, major contributing ancestors, and exotic progenitors of prominent North American potato cultivais. Am J Potato Res 76:263–272.
Lynch, D.R., L.M. Kawchuk, and J. Hachey. 1999. Single gene resistance to verticillium wilt inS. chacoense and incorporation into tetraploid germplasm. 14th Trienn. Conf, Eur Assoc Potato Res, Abstracts, pp. 527-528.
Lynch, D.R., L.M. Kawchuk, J. Hachey, P.S. Bains, and R.J. Howard. 1997. Identification of a gene conferring high levels of resistance to Verticillium wilt inSolanum chacoense. Plant Dis 81:1011–1014.
Mendoza, H.A. 1989. Population breeding as a tool for germplasm enhancement. Am Potato J 66:639–653.
Mendoza, H.A., and F.L. Haynes. 1974. Genetic basis of heterosis for yield in the autotetraploid potato. Theor Appl Gen 45: 21–25.
Müller, KO., and W. Black. 1952. Potato breeding for resistance to blight and virus diseases during the last hundred years. Z Pflanzenz, cht 31:305–318.
Munoz, F.J., and R.L. Plaisted. 1981. Yield and combining abilities in Andigena potatoes after six cycles of recurrent phenotyic selection for adaptation to long day conditions. Am Potato J 58:469- 479.
Murphy, A.M., H.De Jong, and G.C.C. Tai. 1995. Transmission of resistance to common scab from the diploid to the tetraploid level via 4x-2x crosses in potatoes. Euphytica 82:227–233.
Novy, R.G., G.A. Secor, B.L. Farnsworth, J.H. Lorenzen, E.T. Holm, D.A. Preston, N.C. Gudmestad, and J.R. Sowokinos. 1998. NorValley: A white-skinned chipping cultivar with cold-sweetening resistance. Am J Potato Res 75: 101–105.
Pavek, J.J., and D.L. Corsini. 1997. A86SXD6-7: A cold chipping diploid parent with 2N pollen. Am Potato J 74:454. Abstr.
Peloquin, S.J., S.A. Hermunstad, C.O. Okwuagwu, and J.E. Chujoy. 1984. 9thTrienn Conf, Eur Assoc Potato Res. Abstracts p.54.
Peloquin, S.J., S.H. Jansky, and G.L. Yerk. 1989. Potato cytogenetics and germplasm utilization. Am Potato J 66:629–638.
Plaisted, R.L., D.E. Halseth, B.B. Brodie, S.A. Slack, J.B. Sieczka, B.J. Christ, K.M. Paddock, and M.W. Peck. 2001. Eva: A midseason golden nematode- and virus-resistant variety for use as tablestock or chipstock. Am J Potato Res 78:65–68.
Plaisted, R.L., and R.W. Hoopes. 1989. The past record and future prospects for the use of exotic potato germplasm. Am Potato J 66:603–627.
Plaisted, R.L, H.D. Thurston, J.B. Sieczka, B.B. Brodie, E.D. Jones, R.C. Cetas. 1981. Rosa: a new golden nematode resistant variety for chipping and tablestock. Am Potato J 58:451–455.
Plaisted, R.L., W.M. Tingey, and J.C. Steffens. 1992. The germplasm release of NYL235-4, a clone with resistance to the Colorado Potato Beetle. Am Potato J 69:843–846.
Ramon, M., and R.E. Hanneman Jr. 1996. Introgression of resistance to late blight from wild species using embryo rescue and double pollination. Am Potato J 73:379.1996. Abstr.
Rasco Jr., E.T. R.L. Plaisted, and E.E. Ewing. 1980. Photoperiod response and earliness ofS. tuberosum ssp.andigena after six cycles of recurrent selection for adaptation to long days. Am Potato J 57:435–447.
Ross, H. 1979. Wild species and primitive cultivars as ancestors of potato varieties.In: Proc. Int. Conf. Broadening Genetical Base of Crops, Wageningen; Pudoc, Wageningen, 1978. pp. 237- 245.
Ross, H. 1986. Potato breeding—Problems and perspectives. Advances in plant breeding. 132 p. Supplement 13 to Journal of Plant Breeding. Paul Parey, Berlin and Hamburg, Publisher.
Salaman, R.N. 1937. The potato in its early home and its introduction into Europe. J Royal Hort Soc 62:253–266.
Sanford, J.C, and R.E. Hanneman Jr. 1982. A possible heterotic threshold in the potato and its implications for breeding. Theor Appl Gen 61:151–159.
Sanford, L.L., R.S. Kobayashi, K.L. Deahl, and S.L.Sinden. 1997. Diploid and tetraploidSolanum chacoense genotypes that synthesize leptine glycoalkaloids and deter feeding by Colorado Potato Beetle. Am Potato J 74:15–21.
Świezyński, K.M. 1990. Resistance toPhytophthora infestans in the potato. Institute for Potato Research, Bonin, Poland. 76 p.
Tarn, T.R., and G.C.C. Tai. 1977. Heterosis and variation of yield components in F1 hybrids between group Tuberosum and group Andigena potatoes. Crop Sci 17:517–521.
Tarn, T.R., and G.C.C. Tai. 1983. Tuberosum x Tuberosum and Tuberosum x Andigena potato hybrids: comparisons of families and parents, and breeding strategies for Andigena potatoes in long-day temperate environments. Theor Appl Gen 66:87–91.
Thill, CA., and S.J. Peloquin. 1995. Tetraploid potato clones with 25% wild species germplasm that produce cold (4C) chipping progenies. Am Potato J 72: 659–660. Abstr.
Thill, C, and S.J. Peloquin. 1999. The identification of superior parents having 25%Solanum tarijense and used to develop cold-chipping progeny. 14th Trienn Conf, Eur Assoc Potato Res. Abstracts, pp. 327-328.
Vavilov, N.I. 1951. The origin, variation, immunity, and breeding of cultivated plants. Chronica Botanica 13:1–366. Translated from Russian. (Quoted by Hawkes (1990) on p. 200.)
Author information
Authors and Affiliations
Corresponding author
Additional information
Expanded from the 1999 PAA 83Prd annual meeting symposium presentation.
Rights and permissions
About this article
Cite this article
Pavek, J.J., Corsini, D.L. Utilization of potato genetic resources in variety development. Am. J. Pot Res 78, 433–441 (2001). https://doi.org/10.1007/BF02896375
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02896375