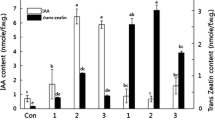
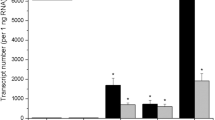
Abstract
On the basis of earlier data it was suggested that the induction of cytokinin autonomy might be accompanied by disorders in plastid function and a decrease in cytokinin utilization. In the work presented below the formation of chlorophyll and the isozyme patterns of nine enzymes, some of which are known to be localized in plastids, were compared in tobacco callus tissues differing in their hormonal requirements. Tissues either not requiring cytokinin or both auxin and cytokinin for their growth, contained a lower amount of chlorophyll than the cytokinin-and auxin-dependent strain. The number of isozymes of glucose-6-phosphate and NADP-malate dehydrogenase (i.e. enzymes which are known to be located in plastids) was reduced from four in the cytokinin-and auxin-dependent strain to two and one in the two cytokinin-autonomous strains, respectively. The fully habituated tissue contained an additional isozyme of NADP-malate dehydrogenase. The total number of isozymes of the remaining enzymes (NAD-malate dehydrogenase, peroxidase, esterase and a-and β-galactosidase) either was decreased or not changed in the cytokinin autonomous strains. The exception was an additional anodic peroxidase in one strain. The number of these isozymes in tissue habituated with respect to both auxin and cytokinin either remained the same or increased. Tobacco callus strains with altered requirements for growth regulators contained some new isozymes which were not present in any other strain and some isozymes present in other strains were absent. These differences are discussed in relation to the possible role of plastid function disorder associated with habituation.
Similar content being viewed by others
Abbreviations
- D:
-
cytokinin- and auxin-dependent tobacco callus strain
- AFH:
-
fully habituated, i.e. cytokinin- and auxin-autonomous tobacco callus strain
- As and A2,4-D:
-
cytokinin-autonomous tobacco callus strains obtained after treatment of D tissue with streptomycin and 2,4-D, respectively
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- NAD-MDH and NADP-MDH:
-
malate dehydrogenase dependent on NAD and NADP, respectively
- G6PDH:
-
glucose-6-phosphate dehydrogenase
References
Bednar, T. W., Linsmaier-Bednar, E. M., King, C. M.: Peroxidase-H2O2 catalyzed incorporation of auxin derivatives into sRNA. -Biochem. biophys. Res. Commun.72: 761–767, 1976.
Beneš, K., Georgieva, Y. D., Poláčková, D.: The presence and distribution of α-and β--glucosidase in root tip. -Biol. Plant.15: 88–94, 1973.
Bouchet, M., Gaspar, Th., Thorpe, T. A.: Soluble and cell-wall peroxidases and auxin destruction in normal and habituated tobacco callus. -In Vitro14: 819–823, 1978.
Braun, A. C.: A physiological bases for autonomous growth of the crown-gall tumor cell. -Proc. nat. Acad. Sci. U.S.A.44: 344–349, 1958.
Buiatti, M., Bennici, A.: Callus formation and habituation inNicotiana species in relation to the specific ability for differentiation. -Acad. Nazionale dei Lincei, Rendiconti Classe Sci. fisiche, matem. e naturali Ser. VIII,48: 261–269, 1970.
Davis, D. J.: Disc electrophoresis: II. Method and application to human serum proteins. -Ann. N.Y. Acad. Sci.121: 407–427, 1964.
Fottrell, F. F.: Esterase isozymes from legume root nodules. -Phytochemistry7: 23–29, 1968.
Hadačová, V., Kamínek, M., Luštinec, J.: Glusoce-6-phosphaee dehydrogenase in tobacco callus strains differing in their growth and their requirement for auxin and cytokinin. -Biol. Plant.17: 448–451, 1975.
Hallam, N. D.: The effect of 2,4-dichlorophenoxyacetic acid and related compounds on the fine structure of primary leaves ofPhaseolus vulgaris. -J. exp. Bot.21: 1031–1038, 1970.
Hallam, N. D., Sargent, J. A.: The localization of 2,4-D in leaf tissue. -Planta94: 291–329, 1970.
Hatch, M. D., Slack, C. R.: NADP specific malate dehydrogenase and glycerate kinase in leaves and evidence for their location in chloroplasts. -Biochem. biophys. Res. Commun.34: 589–593, 1969.
Hoover, J. D., Wender, S. H., Smith, E. C.: Isozymes of glucose-6-phosphate dehydrogenase from tobacco cells. -Phytochemistry16: 195–197, 1977.
Kamínek, M., Luštinec, J.: Reduced chlorophyll synthesis in cytokinin-autonomous strains of tobacco callus. -Z. Pflanzenphysiol.73: 65–73, 1974a.
Kamínek, M., Luštinec, J.: Induction of cytokinin-autonomy and chlorophyll-deficiency in tobacco callus tissue by streptomycin. -Z. Pflanzenphysiol.73: 74–81, 1974b.
Kamínek, M., Luštinec, J.: Induction of cytokinin-autonomy in tobacco stem pith tissue by 2,4-dichlorophenoxyacetic acid. -In:Schreiber, K., Schütte, H. R., Sembdner, G. (ed.): Proc. Internat. Symp. “Biochemistry and Chemistry of Plant Growth Regulators”, Cottbus. Pp. 307–310. Acad. Sci. GDR Halle (Saale) 1974c.
Lee, T. T.: Cytokinin-controlled indoleacetic acid oxidase isozymes in tobacco callus cultures. -Plant Physiol.47: 181–185, 1972a.
Lee, T. T.: Changes in indoleacetic acid oxidase isozymes in tobacco tissue after treatment with 2,4-dichlorophenoxyacetic acid.-Plant Physiol.49: 957–960, 1972b.
Lee, T. T.: Cytokinin control in subcellular localization of indoleacetic acid oxidase and peroxidase. -Phytochemistry13: 2445–2453, 1974.
Lescure, A. M.: Chloroplast differentiation in cultured tobacco cells:In vitro protein synthesis efficiency of plastids at various stages of their evolution. -Cell Differentiation7: 139–152, 1978.
Linsmaier, E. M., Skoog, F.: Organic growth factor requirements of tobacco tissue cultures. -Physiol. Plant.18: 100–127, 1965.
Mäder, M., Meyer, Y., Bopp, M.: Localization dor Peroxidase-isozyme in Protoplasten und Zellwänden vonNicotiana tabacum L. -Planta122: 259–268, 1971.
Nadakavukaren, M. J., McCracken, D. A.: Effect of 2,4-dichlorophenoxyacetic acid on the structure and function of developing chloroplasts. -Planta137: 65–69, 1977.
Novacký, A., Hampton, R. E.: The effect of substrate concentration on the visualization of peroxidases in disc electrophoresis. -Phytochemistry7: 1143–1145, 1968.
Reisfeld, R. A., Lewis, V. J., Williams, D. E.: Disc electrophoresis of basic proteins and peptides on polyacrylamide gels. -Nature195: 281–283, 1962.
Sacristán, M., Melchers, G.: Regeneration of plants from “habituated” and “Agrobacterium--transformed” single-cell clones of tobacco. -Molec. gen. Genet.152: 111–117, 1977.
Sahulka, J., Beneš, K.: Fractions of non-specific esterase in root tip ofVicia faba L. revealed by disc electrophoresis in acrylamide gel. -Biol. Plant.11: 23–33, 1969.
Schrauwen, J.: Nachweis von Enzymen nach electrophoretischer Trennung an Polyacrylamidsäulchen. -J. Chromatogr.23: 117–180, 1966.
Seyer, P., Marty, D., Lescurf, A. M., Péaud-Lenoël, C: Effect of cytokinin on chloroplast cyclic differentiation in cultured tobacco cells. -Cell Differentiation4: 187–197, 1975.
Shinshi, H., Noguchi, M.: Relationships between peroxidase, IAA oxidase and polyphenol oxidase.-Phytochemistry14: 1255–1258, 1975.
Vyskot, B., Novák, F.: Habituation and organogenesis in callus cultures of chlorophyll mutants ofNicotiana tabacum L. -Z. Pflanzenphysiol.81: 34–42, 1977.
Washitani, I., Sato, S.: Studies on the function of proplastids in the metabolism ofin vitro cultured tobacco cells. I. Localization of nitrite reductase and NADP-dependent glutamate dehydrogenase. -Plant Cell Physiol.18: 117–125, 1977a.
Washitani, I., Sato, S.: Studies on the function of proplastids in the metabolism ofin vitro- cultured tobacco cells. III. Source of reducing power for amino acid synthesis from the nitrite. -Plant Cell Physiol.18: 1235–1241, 1977b.
Zamski, E., Umiel, N.: Streptomycin resistance in tobacco: II. Effect of drug on the ultrastructure of plastids and mitochondria in callus cultures. -Z. Pflanzenphysiol.88: 317–325, 1978.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kamínek, M., Hadačová, V. & Luštinec, J. Origin of cytokinin-and auxin-autonomy and changes in specific proteins in tobacco callus tissue. Biol Plant 23, 228–236 (1981). https://doi.org/10.1007/BF02894894
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02894894