Abstract
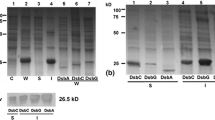
Carcinoembryonic antigen (CEA), the most widely used human tumor marker, is a heavily glycosylated protein over-expressed by a wide range of tumors. It has been indicated that CEA might be a useful target for human anti-tumor immunotherapy. CEA assay for research as well as clinical trials demands a continuous source of CEA protein preparations. In a multi-purpose research program to provide a reliable source for large production of CEA, we converted the membrane-bound carcinoembryonic antigen into a secretory protein by site-specific mutagenesis. We made the secretory CEA protein by introducing a new stop codon at 99 bp upstream of the original stop codon in CEA cDNA by PCR-based mutagenesis. The glycosylation of recombinant CEA proteins, especially those destined for administration to human trials is crucially important. To produce CEA with the same glycosylation pattern and immunogenicity as the native CEA expressed by human tumorsin vivo, the truncated CEA cDNA which does not encode the last C-terminal 33-amino acid hydrophobic domain was transfected into HT29, a human colon carcinoma cell line by the calcium phosphate method. Stable transfectants were selected and pooled. CEA secretion from the cells was verified by analysis of the transfectant culture supernatant for CEA protein. As determined by ELISA, 16 μg/L of recombinant CEA was secreted per 106 transfectants within 48 hrs, an increase over 40 times relative to the untransfected cells. The size of the recombinant CEA secreted by HT29 transfectants in our experiment is identical to that of reference CEA secreted from tumors and is fully antigenic. It seems that the C-terminal truncation does not affect CEA glycosylation in HT29 cells. It is predicted that human cancer immunotherapy using recombinant CEA expressed in this system would be more effective than the commercial protein which is usually prepared from bacterial or other heterologous expression systems.
Similar content being viewed by others
References
Benchimol S, Fuks A, Jothy S, et al: Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 57: 327–334, 1989.
Berinstein NL: Carcinoeinbryonic antigen as a target for therapeutic anticancer vaccines: a review. J Clin Oncol 20: 2197–2207, 2002.
Bohme U, Cross GA: Mutational analysis of the variant surface glycoprotein GPI-anchor signal sequence in Trypanosoma brucei. J Cell Sci 115: 805–816, 2002.
Chowdhury S, Chester KA, Bridgewater J, et al: Efficient retroviral vector targeting of carcinoembryonic antigen positive tumors. Mol Ther 9: 85–92, 2004.
Ford CH, Macdonald F, Griffin JA, et al: Immunoadsorbent purification of carcinoembryonic antigen using a monoclonal antibody: a direct comparison with a conventional method. Tumour Biol 8: 241–250, 1987.
Garcia M, Seigner C, Bastid C, et al: Carcinoembryonic antigen has a different molecular weight in normal colon and in cancer cells due to N-glycosylation differences. Cancer Res 51: 5679–5686, 1991.
Gouin E, Ouary M, Pogu S, et al: Release of carcinoembryonic antigen from human tumor cells by phosphatidylinositol-specific phospholipase C: highly effective extraction and upregulation from LS-174T colonic adenocarcinoma cells. Arch Biochem Biophys 306: 125–132, 1993.
Hefta LJ, Schrewe H, Thompson JA, et al: Expression of complementary DNA and genomic clones for carcinoembryonic antigen and nonspecific cross-reacting antigen in Chinese hamster ovary and mouse fibroblast cells and characterization of the membrane-expressed products. Cancer Res 50: 2397–2403, 1990.
Hefta SA, Hefta LJ, Lee TD, et al: Carcinoembryonic antigen is anchored to membranes by covalent attachment to a glycosylphosphatidylinositol moiety: identification of the ethanolamine linkage site. Proc Natl Acad Sci USA 85: 4648–4652, 1988.
Ikeda S, Kuroki M, Haruno M, et al: Epitope mapping of the carcinoembryonic antigen with various related recombinant proteins expressed in Chinese hamster ovary cells and 25 distinct monoclonal antibodies. Mol Immunol 29:229–240, 1992.
Ilantzis C, DeMarte L, Screaton RA, et al: Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia 4: 151–163, 2002.
Jessup JM, Ishii S, Mitzoi T, et al: Carcinoembryonic antigen facilitates experimental metastasis through a mechanism that does not involve adhesion to liver cells. Clin Exp Metastasis 17:481–488, 1999.
Jessup JM, Petrick AT, Toth CA, et al: Carcinoembryonic antigen: enhancement of liver colonisation through retention of human colorectal carcinoma cells. Br J Cancer 67: 464–470, 1993.
Keep PA, Leake BA, Rogers GT: Extraction of CEA from tumour tissue, foetal colon and patients’ sera, and the effect of perchloric acid. Br J Cancer 37: 171–189, 1978.
Liu KJ, Wang CC, Chen LT, et al: Generation of carcinoembryonic antigen (CEA)-specific T-cell responses in HLA-A*0201 and HLA-A*2402 late-stage colorectal cancer patients after vaccination with dendritic cells loaded with CEA peptides. Clin Cancer Res 10: 2645–2651, 2004.
Kuroki M, Murakami M, Wakisaka M, et al: Immunoreactivity of recombinant carcinoembryonic antigen proteins expressed in Escherichia coli. Immunol Invest 21: 241–257, 1992.
Kuroki M, Kuwahara M, Tsuruta Y, et al: Effective purification of nonspecific cross-reacting antigens with phosphatidylinositol-specific phospholipase C. Prep Biochem 23: 333–349, 1993.
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970.
Leconte A, Garambois V, Ychou M, et al: Involvement of circulating CEA in liver metastases from colorectal cancers reexamined in a new experimental model. Br J Cancer 80: 1373–1379, 1999.
Matsuoka Y, Matsuo Y, Okamoto N, et al: Highly effective extraction of carcinoembryonic antigen with phosphatidylinositol-specific phospholipase C. Tumour Biol 12: 91–98, 1991.
Mayer A, Chester KA, Flynn AA, Begent RH: Taking engineered anti-CEA antibodies to the clinic. J Immunol Methods 231:261–273, 1999.
Nap M, Mollgard K, Burtin P, et al: Immunohistochemistry of carcino-embryonic antigen in the embryo, fetus and adult. Tumour Biol 9: 145–153, 1988.
Rudd PM, Wormald MR, Stanfield RL, et al: Roles for glycosylation of cell surface receptors involved in cellular immune recognition. J Mol Biol 293: 351–366, 1999.
Samanci A, Yi Q, Fagerberg J, et al: Pharmacological administration of grauulocyte/macrophage-colony-stimulating factor is of significant importance for the induction of a strong humoral and cellular response in patients immunized with recombinant carcinoembryonic antigen. Cancer Immunol Immunother 47: 131–142, 1998.
Sarobe P, Huarte E, Lasarte JJ, et al: Carcinoembryonic antigen as a target to induce anti-tumor immune responses, Review. Curr Cancer Drug Targets 4: 443–454, 2004.
Shively JE, Beatty JD: CEA-related antigens: molecular biology and clinical significance. Crit Rev Oncol Hematol 2: 355–399, 1985.
Terskikh A, Mach JP, Pelegrin A: Marked increase in the secretion of a fully antigenic recombinant carcinoembryonic antigen obtained by deletion of its bydrophobic tail. Mol Immunol 30: 921–927, 1993.
Udenfriend S, Micanovic R, Kodukula K: Structural requirements of a nascent protein for processing to a PI-G anchored form: studies in intact cells and cell-free systems. Cell Biol Int Rep 15: 739–759, 1991.
Udenfriend S, Kodukula K: How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu Rev Biochem 64: 563–591, 1995.
Ullenhag GJ, Frodin JE, Jeddi-Tehrani M, et al: Durable carcinoembryonic antigen (CEA)-specific humoral and cellular immune responses in colorectal carcinoma patients vaccinated with recombinant CEA and granulocyte/macrophage colony-stimulating factor. Clin Cancer Res 10: 3273–3281, 2004.
Witherspoon LR, Shuler SE, Alyea K, et al: Carcinoembryouic antigen: assay following heat compared with perchloric acid extraction in patients with colon cancer, non-neoplastic gastrointestinal diseases, or chronic renal failure. J Nucl Med 24:916–921, 1983.
Wong JY, Chu DZ, Williams LE, et al: Pilot trial evaluating an 1231-labeled 80-kilodalton engineered anticarcinoembryonic antigen antibody fragment (cT84.66 minibody) in patients with colorectal cancer. Clin Cancer Res 10: 5014–5021, 2004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naghibalhossaini, F., Pakdel, A., Ghaderi, A.A. et al. Effective production of carcinoembryonic antigen by conversion of the membrane-bound into a recombinant secretory protein by site-specific mutagenesis. Pathol. Oncol. Res. 11, 211–217 (2005). https://doi.org/10.1007/BF02893853
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02893853