Abstract
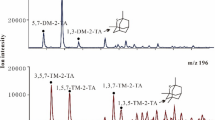
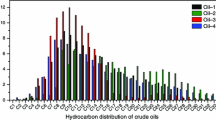
The mechanism of carbon isotopic fractionation for gaseous hydrocarbons is revealed by investigating the residual liquid hydrocarbons in laboratory pyrolysates of n-octodecane. The results indicate that cracking and polymerization in the relatively low temperatures and disproportionation reactions leading to light hydrocarbons and polyaromatic hydrocarbons at high temperatures are probably causes for the carbon isotope reversal of gaseous hydrocarbons that is commonly observed in pyrolysis experiments. This study provides significant insight for quantitative modeling of natural gas δ13C values and aid in the identification and assessment of natural gases derived from oil cracking.
Similar content being viewed by others
References
Horsfield, B., Schenk, H. J., Mills, N. et al., An investigation of the in-reservoir conversion of oil to gas: compositional and kinetic findings from closed-system programmed-temperature pyrolysis, Org. Geochem., 1992, 19: 191–204.
Ungerer, P., Behar, F., Villalbe, M. et al., Kinetic modeling of oil cracking, Org. Geochem., 1988, 13: 857–868.
Sackett, W. M., Nakaparksin, S., Dalrymple, D., Carbon isotope effects in methane production by thermal cracking, In Advances in Organic Geochemistry, 1966 (eds. Hobson, G. D., Speers, G. C.), Oxford: Pergamon Press, 1968, 37–53.
Sackett, W. M., Carbon and hydrogen isotope effects during the thermocatalytic production of hydrocarbons in laboratory simulation experiments, Geochimica et Cosmochimica Acta, 1978, 42: 571–580.
Cramer, B., Krooss, B. M., Littke, R., Modeling isotope fractionation during primary cracking of natural gas: A reaction kinetic approach, Chem. Geol., 1998, 149: 235–250.
Lorant, F., Prinzhofer, A., Behar, F. et al., Carbon isotopic and molecular constraints on the formation and the expulsion of thermogenic hydrocarbon gases, Chem. Geol., 1998, 147: 249–264.
Xiong, Y. Q., Geng, A. S., Liu, J. Z., Kinetic simulating experiment combined with GC-IRMS analysis: Application to identification and assessment of coal-derived methane from Zhongba Gas Field (Sichuan Basin, China), Chemical Geology, 2004, 213(4): 325–338.
Behar, F., Kressmann, S., Vandenbroucke, M. et al., Experimental simulation in a confined system and kinetic modeling of kerogen and oil cracking, Org. Geochem., 1991, 19: 173–189.
Behar, F., Vandenbroucke, M., Tang, Y. et al., Thermal cracking of kerogen in open and closed systems: determination of kinetic parameters and stoichiometric coefficients for oil and gas generation, Org. Geochem., 1997, 26(5/6): 321–339.
Frank, D. J., Sackett, W. M., Kinetic isotope effects in the thermal cracking of neopentane, Geochimica et Cosmochimica Acta, 1969, 33: 811–820.
Behar, F., Budzinski, H., Vandenbroucke, M. et al., Methane generation from oil cracking: kinetics of 9-methylphenanthrene cracking and composition with other pure compounds and oil fractions, Energy & Fuels, 1999, 13: 471–481.
Lorant, F., Behar, F., Vandenbroucke, M., Methane generation from methylated aromatics: kinetic study and carbon isotope modeling, Energy & Fuels, 2000, 14: 1143–1155.
Xiong, Y. Q., Geng, A. S., Wang, Y. P. et al., Kinetic simulating experiment on the secondary hydrocarbon generation of kerogen, Science in China, Series D, 2002, 45(1): 13–20.
Tang, Y., Perry, J. K., Jenden, P. D. et al., Mathematical modeling of stable carbon isotope ratios in natural gases, Geochimica et Cosmochimica Acta, 2000, 64: 2673–2687.
Cramer, B., Faber, E., Gerling, P. et al., Reaction kinetics of stable carbon isotopes in natural gas-insights from dry, open system pyrolysis experiments, Energy & Fuels, 2001, 15: 517–532.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xiong, Y., Zhang, H., Geng, X. et al. Thermal cracking ofn-octodecane and its geochemical significance. Chin. Sci. Bull. 49 (Suppl 1), 79–83 (2004). https://doi.org/10.1007/BF02890457
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02890457