Summary
Adult male untreated mice (NMRI) were investigated after radioactive labeling with3H-thymidine and3H-deoxycytidine to find out whether the lymphocytes in the cortex and medulla of the thymus as well as in the perifollicular and periarteriolar regions of the spleen show a labeling pattern which allows a classification into T- and B-lymphocytes. The percentages of radioactively labeled small lymphocytes and their mean grain counts were determined.
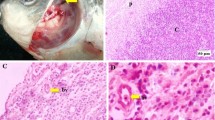
The percentages of radioactively labeled small lymphocytes after3H-TdR and3H-CdR showed no significant differences in both splenic zones. The grain counts over the lymphocyte nuclei in the periarteriolar zone showed lower values after3H-TdR than after3H-CdR. The lymphocytes in the perifollicular zone were strongly labeled with3H-TdR and weakly labeled with3H-CdR. In the thymus medulla, lymphocytes were weakly labeled with3H-thymidine and strongly labeled with3H-CdR. In the cortex no significant differences were observed. 75 to 80% of the small lymphocytes in the peripheral blood were weakly and 20–25% strongly labeled after3H-TdR.
Therefore there are similarities in the radioactive labeling pattern of thymic medulla lymphocytes and that of small lymphocytes of the periarteriolar zone of the spleen by both DNA precursors. The small lymphocytes in the peripheral T-dependent tissue zones, for example in the spleen, as well as in the mixed lymphocyte population of the peripheral blood can be differentiated from the B-lymphocytes through the difference in the amount of incorporation of3H-thymidine and3H-deoxycytidine.
Similar content being viewed by others
References
Amano, M., Everett, N.B.: Preferential labeling of rat lymphocytes with a rapid rate of turnover by tritiated deoxycytidine. Cell Tiss. Kinet.9, 167–177 (1976)
Brahim, F., Osmond, D.O.: Migration of bone marrow lymphocytes demonstrated by selective bone marrow labeling with thymidine H3. Anat. Rec.168, 139–160 (1970)
Bryant, B.J., Hess, M.W., Cottier, H.: Thymus lymphocytes, efflux and restoration phases after peripheral exposure to phytohaemagglutin, Immunol.29, 115–120 (1975)
Biirki, H., Cottier, H., Hess, M.W., Laissue, J., Stoner, R.D.: Distinctive medullary and germinal center proliferative patterns in mouse lymph nodes after regional primary and secondary stimulation with tetanus toxoid. J. Immunol.112, 1961–1970 (1974)
Cohen, J.J., Fischbach, M.C., Claman, H.N.: Hydrocortisone resistance of graft-versus-host activity in mouse thymus, spleen and bone marrow. J. Immunol.105, 1146–1150 (1970)
Cottier, H., Hess, M.W., Roos, B., Gretillat, P.A.: Regeneration, Hyperplasie und Onkogenese der lymphoretikulären Organe. In Hdb. Allgemeine Pathologie (Hrsg. H.W. Altmann, F. Büchner, H. Cottier, E. Grundmann, G. Holle, E. Letterer, W. Masshoff, H. Meessen, F. Roulet, G. Seifert, G. Siebert, A. Studer) VI/2 496–709. Berlin, Heidelberg, New York, Springer (1969)
Cottier, H., Schindler, R., Bürki, H., Sordat, B., Joel, D.D., Hess, M.W.: Kinetic aspects of lymphocyte recirculation. Int. Arch. Allerg.41, 4–12 (1971)
Cottier, H., Hess, M.W., Schädeli, J., Bürki, H.: Lymphocytenformen: Herkunft und Entwick-lungsmöglichkeiten. Verhdl. Dtsch. Ges. Inn. Med.79, 99–105 (1973)
Craddock, C.G., Longmire, R., McMillan, R.: Lymphocytes and the immune response. First of two parts. New Engl. J. Med.285, 324–331 (1971a)
Craddock, C.G., Longmire, R., McMillan, R.: Lymphocytes and the immune response. New Engl. J. Med.285, 378–384 (1971b)
Everett, N.B., Caffrey, R.W., Rieke, W.O.: Recirculation of lymphocytes. Ann. New. York Acad. Sc.113, 887–897 (1964)
Feinendegen, L.E.: Tritium-labeled molecules in biology and medicine. New York: Acad. Press 1967
Feinendegen, L.E., Heiniger, H.J., Friedrich, G., Cronkite, E.P.: Differences in reutilization of thymidine in hemopoietic and lymphopoietic tissues of the normal mouse. Cell Tiss. Kinet.6, 573–585 (1973)
Ford, W.L.: The kinetics of lymphocyte recirculation within the rat spleen. Cell Tiss. Kinet.2, 171–191 (1969a)
Ford, W.L.: The immunological and migratory properties of the lymphocytes recirculating through the rat spleen. Brit. J. Exp. Path.50, 257–269 (1969b)
Ford, W.L.: Lymphocyte migration and immune responses. Prog. Allergy19, 1–59 (1975)
Ford, W.L., Gowans, J.L.: The traffic of lymphocytes. Sem. Hemat.6, 67–83 (1969)
Goldschneider, I.: Antigenic relationship between medullary thymocytes and a subpopulation of peripheral T cells in the rat. Description of a masked antigen. Cell Immunol.16, 269–284 (1975)
Goldschneider, I., Cogen, R.B.: Immunoglobulin molecules on the surface of activated T lymphocytes in the rat. J. Exp. Med.138, 163–175 (1973)
Gowans, J.L.: Lifespan, recirculation, and transformation of lymphocytes. Int. Rev. Exp. Path.5, 1–21 (1966)
Gowans, J.L., McGregor, D.D.: The immunological activities of lymphocytes. Prog. Allergy9, 1–78 (1965)
Gowans, J.L., Knight, E.J.: The route of recirculation of lymphocytes in the rat. Proc. Roy. Soc B159, 257–282 (1964)
Greaves, M.F., Janossy, G.: Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transpl. Rev.11, 87–130 (1972)
Griss, P., Heitmann, K., Wegener, K., Ditzen, K.: Zur Topik der Thymozytenproliferation nach akzidenteller Involution. Verh. Dtsch. Ges. Path.54, 222–228 (1970)
Grouls, V., Helpap, B.:3H-Thymidin-Inkorporation in Lymphozyten des Thymus nach Thermonekrosen an inneren Organen. Verhdl. Dtsch. Ges. Path.61, 379 (1977)
Gutman, G.A., Weissman, I.L.: Lymphoid tissue architecture: Experimental analysis of the origin and distribution of T cells and B cells. Immunol.23, 464–478 (1972)
Helpap, B., Grouls, V., Yamashita, K., Breining, H.: The proliferative response of the spleen in cryosurgery. Cryobiology13, 54–60 (1976)
Helpap, B., Grouls, V., Breining, H.: The splenic reaction to cryosurgical lesions on parenchymal organs. Naturwissenschaften64, 647 (1977)
Hoffert, J.F., White, A.: Effect of a single injection of cortisol on the incorporation of3H-thyraidine and3H-deoxycytidine into lymphatic tissue DNA of adrenalectomized rats. Endocrinology82, 767–776 (1968)
Hollingsworth, J.W., Carr, J.: Migration of rat thoracic duct lymphocytes from tissues to central lymphatic circulation. Cell Immunol.5, 228–232 (1972)
Hollingsworth, J.W., Carr, J.:3H-Uridine incorporation as a T lymphocyte indicator in the rat. Cell Immunol.8, 270–279 (1973)
Howard, J.C.: The life-span and recirculation of marrow derived small lymphocytes from the rat thoracic duct. J. Exp. Med.135, 185–199 (1972)
Howard, J.C., Hunt, S.V., Gowans, J.L.: Identification of marrow-derived and thymus-derived small lymphocytes in the lymphoid tissue and thoracic duct lymph of normal rats. J. Exp. Med.135, 200–219 (1972)
Howard, J.C., Scott, D.W.: The identification of sera distinguishing marrow-derived and thymusderived lymphocytes in the rat thoracic duct. Immunol.27, 903–922 (1974)
Hwang, W.S., Ho, T.Y., Luk, S.C., Simon, G.T.: Ultrastructure of the rat thymus: a transmission, scanning electron microscope, and morphometric study. Lab. Invest.31, 473–487 (1974)
Joel, D.D., Hess, M.W., Cottier, H.: Magnitude and pattern of thymic lymphocyte migration in neonatal mice. J. Exp. Med.135, 907–923 (1972)
Jondal, M., Holm, G., Wigzell, H.: Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J. Exp. Med.136, 207–215 (1972)
Jondal, M., Pross, H.: Surface markers on human B and T lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int. J. Cancer15, 596–605 (1975)
Kruyff de, R.H., Durkin, H.G., Gilmour, D.G., Thorbecke, G.J.: Migratory patterns of B lymphocytes. II. Fate of cells from central lymphoid organs in the chicken. Cell. Immunol.16, 301–314 (1975)
Lennert, K., Müller-Hermelink, H.K.: Lymphocyten und ihre Funktionsformen. Morphologie, Organisation und immunologische Bedeutung. Verh. Anat. Ges.69, 19–62 (1975)
Lin, P.S., Cooper, A.G., Wortis, H.H.: Scanning electron microscopy of human T cell and B cell rosettes. New Engl. J. Med.289, 548–551 (1973)
Matter, A.: Morphological definition of thymus subpopulations Cell Tiss. Res.158, 319–338 (1975)
Matter, A., Lisowska-Bernstein, B., Ryser, J.E., Lamelin, J.P., Vassalli, P.: Mouse thymus-independent and thymus-derived lymphoid cells. II. Ultrastructural studies. J. exp. Med.136, 1008–1030 (1972)
Miller, J.F.A.P.: Immunological function of the thymus. Lancet II, 748–764 (1961)
Mitchell, G.J.: Lymphocyte circulation in the spleen. Marginal zone bridging channels and their possible role in cell traffic. Immunol.24, 93–107 (1973)
Nieuwenhuis, P., Ford, W.L.: Comparative migration of T- and B-lymphocytes in the rat spleen and lymphnodes. Cell Immunol.23, 254–267 (1976)
Nieuwenhuis, P., Keuning, F. J.: Germinal centres and the origin of the B cell system. II. Germinal centres in the rabbit spleen and popliteal lymphnodes. Immunol.26, 509–519 (1974)
Nieuwenhuis, P., Nouhuijs, C.E., van Eggens, J.H., Keuning, F.J.: Germinal centres and the origin of the B cell system. I. Germinal centres in the rabbit appendix. Immunol.26, 497–508 (1974)
Osogoe, B., Ueki, A.: A radioautographic study of the utilization of deoxycytidine for the formation of deoxyribonucleic acid thymine in lymphocytes. J. Cell Biol.46, 403–405 (1970)
Osogoe, B., Tyler, R.W., Everett, N.B.: The patterns of labeling of germinal-center cells with tritiated deoxycytidine. J. Cell Biol.57 215–220 (1973)
Pabst, R., Trepel, F.: The predominant role of the spleen in lymphocyte recirculation. I. Homing of lymphocytes to and release from the isolated perfused pig spleen. Cell Tiss. Kinet.8, 529–541 (1975)
Pabst, R., Munz, D., Trepel, F.: Splenic lymphocytopoiesis and migration pattern of splenic lymphocytes. Cell Immunol.33, 33–44 (1977)
Parrott, D.M.V., de Sousa, M.A.B.: Thymus-dependent and thymus-independent populations: Origin, migratory patterns, and life-span. Clin. Exp. Immunol.8, 663–684 (1971)
Polliack, A., Fu, S.M., Douglas, S.D., Bentwich, Z., Lampen, N., de Harven, E.: Scanning electron microscopy of human lymphocyte-sheep erythrocyte rosettes. J. Exp. Med.140, 146–158 (1974)
Polliack, A., Lampen, N., Clarkson, B.D., de Harven, E., Bentwich, Z., Siegal, F.P., Kunkel, H.G.: Identification of human B and T lymphocytes by scanning electron microscopy. J. Exp. Med.138, 607–624 (1973)
Raff, M.C.: Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant. Rev.6, 52–80 (1971)
Raff, M.C., Wortis, H.H.: Thymus dependence of Theta-bearing cells in the peripheral lymphoid tissues of mice. Immunol.18, 931–942 (1970)
Rooijen van, N.: Labelling of lymphocytes with various radioisotopes for in vivo tracer studies; a review. J. Immunol. Meth.15, 267–277 (1977)
Röpke, C., Everett, N.B.: Small lymphocyte populations in the mouse bone marrow. Cell Tiss. Kinet.6, 499–507 (1973)
Schädeli, J., Schürch, B.: Minimal intrathymic lymphoid cell death in specific pathogen-free mice during the perinatal period. Inaug. Diss. Bern 1973
Scott, D.W., Josephs, S.H.: Uridine labelling of human lymphocytes: Differential uptake by T and B cells. Cell immunol.20, 64–68 (1975)
Sprent, J.: Circulating T and B lymphocytes in the mouse. I. Migratory properties. Cell Immunol.7, 10–39(1973)
Sprent, J., Basten, A.: Circulating T and B lymphocytes of the mouse. II. Lifespan. Cell Immunol.7, 40–59 (1973)
Sugino, Y., Frenkel, E.P., Potter, R.L.: Effect of X-radiation on DNA metabolism in various tissues of the rat. V. DNA metabolism in regenerating thymus. Rad. Res.19, 682–700 (1963)
Sullivan, A.K., Adams, L.S., Silke, I., Jerry, L.M.: “Hairy” B cells and “smooth” T cells. New Engl. J. Med.290, 689–690 (1974)
Trepel, F.: Zeilproliferation im lymphatischen Gewebe. Produktion und Verteilung der Lymphozyten. In Der Lymphozyt, Struktur, Physiologie, Pathologie und Klinik (Ed. H. Pietschmann.) pp 127–140. Wien: Med. Akad. Verlag, 1972
Trowell, Q.A.: Radiosensitivity of the cortical and medullary lmphocytes in the thymus. Int. J. Radiat. Biol.4, 163–169 (1961)
Veerman, A.J.P., van Ewijk, W.: White pulp compartments in the spleen of rats and mice. A light and electron microscopic study of lymphoid and non-lymphoid cell types in T and B areas. Cell Tiss. Res.156, 417–441 (1975)
Weissman, I.L.: Thymus cell maturation. Studies on the origin of cortisone-resistant thymic lymphocytes. J. Exp. Med.137, 504–510 (1973)
Wiig, J.N.: Electrophoresis of lymphoid cells. Characterization of T and B lymphocytes and two populations of thymocytes in the mouse. Acta Path. Microbiol. Scand. A Suppl.236, 101–111 (1973)
Zimmermann, A., Brundel, R.G., Bürki, H., Keller, H.-U., Hess, M.W., Cottier, H.: Lymphozyten-formen-Morphologische und funktionelle Charakterisierungsmöglichkeiten, Herkunft und Entwicklung. In: Lymphozyt und Klinische Immunologie (Eds. H. Theml and H. Begemann) pp 2–15. Berlin-Heidelberg-New York: Springer 1975
Author information
Authors and Affiliations
Additional information
Supported by the Deutsche Forschungsgemeinschaft Bonn Bad Godesberg, He 537/5
Rights and permissions
About this article
Cite this article
Helpap, B., Dachselt, U. The pattern of lymphocytes in the thymus and spleen after labeling with3H-thymidine and3H-deoxycytidine. Virchows Arch. B Cell Path. 28, 287–299 (1978). https://doi.org/10.1007/BF02889078
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02889078