Abstract
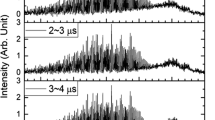
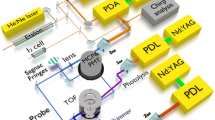
The relaxation of the highly vibrationally excited CO (v = 1–8) by CO2 is studied by timeresolved Fourier transform infrared emission spectroscopy (TR FTIR). 193 nm laser photolysis of the mixture of CHBr3 with O2 generates the highly vibrationally excited CO(v) molecules. TR FTIR records the intense infrared emission of CO(v→v-1). The vibrational populations of each level of CO(v) have been determined by the method of spectral simulation. Based on the evolution of the time resolved populations and the differential method, 8 energy transfer rate constants of CO(v = 1–8) to CO2 molecules areobtained: (5.7±0.1), (5.9±0.1), (5.2±0.2), (3.4±0.2), (2.4±0.3), (2.2±0.4), (2.0±0.4) and (1.8±0.6) (1014 cm3 · molecule−1 · s−1), respectively. A two-channel energy transfer model can explain the feature of the quenching of CO(v) by CO2. For the lower vibrational states of CO, the vibrational energy transfers preferentially to the u3 mode of CO2 For the higher levels, the major quenching channel changes to the vibrational energy exchange between CO(v→v-1) and the u1 mode of CO2.
Similar content being viewed by others
References
Yardley, L. T.,Introduction to Molecular Energy Transfer, New York: Academic Press, 1980, 55–80.
Hancock, G., Smith, I. W. M., Quenching of infrared chemiluminescence, 1: The rate of De-Excitation of CO (4≤v≤13) by He, CO, NO, N2, Q2, OCS, N2O, and CO2,Appl. Opt., 1971, 10(8): 1827.
Caledonia, G. E., Green, B. D., Murphy, R. E., A study of the vibrational level dependent quenching of CO (v = 1–16) by CO2,J. Chem. Phys., 1979, 71: 4369.
Li, H. Z., Wang, X. B., Kong, F. A.et al., The laser photolysis of CHBr3 + O2 Acta Physico-Chimica Sinica (in Chinese), 1993, 9: 452.
Zhu, Q. H., Huang, S. L., Wang, X. B.et al., Time-resolved Fourier transform infrared emission spectroscopy,Chinese J. Chem. Phys. (in Chinese), 1993, 6: 87.
Christoph, M., Michael, J. F., Dwayne, E. H.et al., Rate constants for removal of CH(D)(v = 0 and 1) by collisions with N2, CO, O2. NO and NO2 at 298K and with CO2 at 296≤T/K≤873,J. Chem. Soc., Faraday Trans., 1996, 92 (13): 2335.
Wang, X. B., Li, H. Z., Kong, F. A. et al., The vibrational Quenching of NO (v = 1–11) by N2O studied by time-resolved Fourier transform infrared emission spectroscopy.Chem. Phys. Lett., 1993, 208(3,4); 290.
Miller, D. J., Millikan, R. C., Vibration-vibration energy exchange between carbon monoxide and oxygen,Chem. Phys. Lett., 1974, 27(1): 10.
Green, W. H., Hancock, J. K., Measurement of CO (v = 1) vibrational energy transfer rates using a frequency double CO2 laser,J. Chem. Phys., 1973, 59: 4326.
Millikan, R. C., White, D. R., Systematics of vibrational relaxation,J. Chem. Phys., 1963, 39(12): 3209.
Wang, X. B., Li, H. Z., Kong, F. A.et al., 193 nm photolysis of N2O:The vibrational excited NO(v) and the quenching by N2O,Chinese J. Chem. Phys. (in Chinese), 1993, 6(5): 398.
Schwartz, R. N., Slawsky, Z. I., Herzfeld, K. F., Calculation of vibrational relaxation times in gases,J. Chem. Phys., 1952, 20(10): 1591.
Schwarts, R. N., Herzfeld, K. F., Vibrational relaxation times in gases (three-dimensional treatment),J. Chem. Phys., 1954, 22(5): 767.
Hamzeh, A. H., Ewing, G. E., Vibrational energy transfer between CO and CH4, CD4, CF4 in liquid Ar,J. Chem. Phys., 1985, 82: 5442.
Author information
Authors and Affiliations
About this article
Cite this article
Wang, B., Gu, Y., He, Y. et al. The v-v energy transfer of highly vibrationally excited states (I). Chin. Sci. Bull. 43, 1536–1541 (1998). https://doi.org/10.1007/BF02883444
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02883444