Abstract
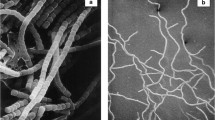
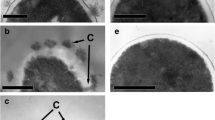
Colonial growth ofNeurospora sitophila phenotypically induced by ramihyphin A is accompanied by marked changes in the contents of DNA, RNA and proteins in the mycelium, and in the relative proportion of hexoses in cell wall hydrolysates. The glucosamine/glucose ratio is also characteristic for colonial growth. X-ray analysis of cell walls showed that ramihyphin A suppresses the crystalline arrangement of chitin in cell walls. A combination of microbiological, biochemical and physico-chemical methods yielded a general picture of the changes accompanying the colonial growth ofNeurospora sitophila.
Similar content being viewed by others
References
Applegarth D. A., Bozoian G.: Presence of chitin in the cell wall of a griseofulvin-producing species ofPenicillium.J. Bacteriol. 94, 1787 (1967).
Ashwell G.: Colorimetric analysis of sugars. In Colowick S. P., Kaplan N. O. (Eds.):Methods in Enzymology, Vol. III, Academic Press, New York—London 1957, p. 87.
Baeáth Z., Babáthová H., Betina V., Nemec P.: Ramihyphins-antifungal and morphogenic antibiotics fromFusarium sp. S-435.Folia Microbiol. 19, 507 (1974).
Baráthová H., Betina V., Baráth Z., Nemec P.: Morphogenic effects of ramihyphin A in filamentous fungi.Folia Microbiol. 20, 97 (1975).
Bartnicki-Garcia S.: Cell wall chemistry, morphogenesis, and taxonomy of fungi.Ann. Rev. Microbiol. 22, 87 (1968).
Bazinet G., Fuscaldo K. E., Lechner J. F.: Analysis of the glucose-6-phosphate dehydrogenase isozymes inNeurospora crassa.Bacteriol. Proc. 1967: 55.
Betina V., Mičeková D.: Morphogenetic activity of cytochalasins, cyanein and monorden inBotrytis cinerea.Z. Allg. Microbiol. 13, 287 (1973).
Betina V., Janstová D., Spišiaková J.: Interferences of antibiotics with the life cycle ofNeurospora crassa.Folia Microbiol. 20, 340 (1975).
Brody S., Tatum E. L.: The primary biochemical effect of a morphological mutation inNeuroepora crassa.Proc. Nat. Acad. Sci. USA 56, 1290 (1966).
Carlström D.: The crystal structure of α-chitin.J. Biophys. Biochem. Gytol. 3, 669 (1957).
Cessi C., Piliego F.: The determination of amino sugars in the presence of amino acids and glucose.Biochem. J. 77, 508 (1960).
Cessi C., Serafini-Cessi F.: A methed for the determination of d-galactosamine in the presence of d-glueosamine.Biochem. J. 88, 132 (1963).
Combépine G., Chervet J.: Preparation of isolated macroconidia ofNeurospora crassa.Experientia 25, 984 (1969).
Grocken B., Tatum E. L.: The effect of sorbose on metabolism and morphology ofNeurospora. Biochim.Biophys. Acta 156, 1 (1968).
Distler J. J., Roseman S.: Galactosamine polymers produced byAspergillus parasiticus.J. Biol. Chem. 235, 2538 (1960).
Glasgow J. E., Reissig J. L.: Interaction of galactosaminoglycan withNeurospora conidia.J. Bacteriol. 120, 759 (1974).
Janson V. K., Nickerson W. J.: Chemical composition of chlamydospores ofCandida albicans.J. Bacteriol. 104, 922 (1970).
Kreger D. R.: Observation on cell walls of yeasts and some other fungi by X-ray diffraction and solubility tests.Biochim. Biophys. Acta 13, 1 (1954).
Lechner J. F., Fuscaldo K. E., Bazinet G.: Genetic and biochemical studies of the hexose monophosphate shunt inNeurospora crassa. II. Characterization of biochemical deffects of the morphological mutants colonial 2 and colonial 3.Canad. J. Microbiol. 17, 789 (1971).
Mahadevan P. R., Mahadkar U. R.: Role of enzymes in growth and morphology ofNeurospora crassa: cell-wall bound enzymes and their possible role in branching.J. Bacteriol. 101, 941 (1970).
Mahadevan P. R., Tatum E. L.: Relationship of the major constituents of theNeurospora crassa cell wall to wild-type and colonial morphology.J. Bacteriol. 90, 1073 (1976).
Martens Ch. L., Sargent M. L.: Circadian rhytms of nucleic acid metabolism inNeurospora crassa.J. Bacteriol. 117, 1210 (1974).
Sohneideb W. C.: Determination of nucleic acids in tissues by pentose analysis. In Colowick S. P., Kaplan N. O. (Eds.):Methods in Snzymology, Vol. III, Academic Press, New York-London 1957, p. 680.
Stewart-Tull D. E. S.: Determination of amino sugars in mixtures containing glucosamine, galactosamine and muramic aoid.Biochem. J. 109, 13 (1968).
De Terra N., Tatum E. L.: A relationship between cell wall structure and colonial growth inNeurospora crassa.Amer. J. Bot. 50, 669 (1963).
Turner W. B., Vogel R.: Vogel’s medium forN. sitophila.J. Bacteriol. 95, 1608 (1968).
Zolokar M.: Growth and differentiation ofNeurospora hyphae.Amer. J. Bot. 46, 602 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Betina, V., Hudec, J., Baráth, Z. et al. Colonial growth and ramihyphin A — Induced changes in cell walls ofNeurospora sitophila . Folia Microbiol 21, 274–284 (1976). https://doi.org/10.1007/BF02876903
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02876903