Abstract
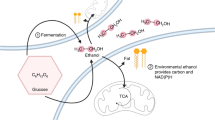
Extrusion of metabolites (glycerol, lactic, malic, and succinic acid) during the medium acidification caused by resting baker’s yeast supplied with 200mm glucose was studied under aerobic and anaerobic conditions and in the absence and presence of 14mm KCl. The maximum levels of glycerol and of the sum of acids (about 13 and 8mm, respectively) were attained anaerobically; aerobiosis reduced the levels by 40–50 % and the presence of K+ ions by another 10–20 %. The time courses of glucose consumption and medium acidification were similar aerobically and anaerobically. The glucose consumption ourves exhibited a short plateau about 2 min after glucose addition, caused probably by a rapid osmotic equilibration of glucose across the cell mambrane. Metabolite extrusion indicates that at high glucose concentrations the alcohol dehydrogenase reaction is supplemented by other reactions aiding in the maintenance of a balanced NAD+/NADH ratio in the cells.
Similar content being viewed by others
References
Aiking H., Sterkenburg A., Tempest D.W.: Influence of specific growth limitation and dilution rate on the phosphorylation efficiency and cytochrome content of mitochondria ofCandida utilis NCY 321.Arch.Microbiol. 113, 65 (1977).
Beown C.M., Johnson B.: Influence of the concentration of glucose and galactose on the physiology ofSaccharomyces cerevisiae in continuous culture.J.Gen.Microbiol. 64, 279 (1970).
Conway E.J., O’Malley E.: The nature of the cation exchanges during yeast fermentation, with formation of 0.02n H ions.Biochem.J. 40, 59 (1946).
Conway E.J., Brady T.G.: Biological production of acid and alkali. I. Quantitative relation of succinic and carbonic acids to the K and H ion exchange in fermenting yeast.Biochem.J. 47, 360 (1950).
Dahlquist A.: Method for assay of intestinal disaccharidases.Anal.Biochem. 7, 18 (1964).
Gancedo C., Gancedo J.M., Sols A.: Glycerol metabolism in yeasts. Pathways of utilization and production.Eur.J.Biochem. 5, 166 (1968).
Harrison D.E.F.: Physiological effects of dissolved oxygen tension and redox potential on growing population of microorganisms.J.Appl.Chem.Biotechnol. 22, 417 (1972).
Holzer H., Bernhardt W., Schneider S.: Zur Glycerinbildung in Bäckerhefe.Biochem.Z. 336, 495 (1963).
Holzer H., Holzer E., Schultz G.: Zusammenhang zwischen Wachstum und aerober Gärung. I. Versuche mit Hefezellen.Biochem.Z. 326, 386 (1965).
Kunkee R.E., Amerine M.A.: Yeast in wine-making, p. 5 inThe Yeasts (A. H, Rose, J.S. Harrison, Eds.). Vol. 3. Academic Press, London-New York 1970.
Ohnishi T., Sottocasa G., Ernster L.: Current approach to the mechanism of energy-coupling in the respiratory chain: Studies with yeast mitochondria.Bull.Soc.Chim.Biol. (Paris) 48, 1189 (1966).
Peňa A.: Studies on the mechanism of K+ transport in yeast.Arch.Biochem.Biophys. 167, 397 (1975).
Peynaud E., Lafon-Lafourcade S., Guimberteau G.: Sur les diverses formes de l’acide lactique dans les milieux fermentés.Compt.rend. 263, 634 (1966).
Polakis E.S., Bartley W., Meek G.A.: Changes in the activities of respiratory enzymes during the aerobie growth of yeast on different carbon sources.Biochem.J. 97, 298 (1965).
Radler F., Fuck E.: Die Umsetzung von L-Apfelsäure durchSaccharomyces cerevisiae bei der Gärung.Experientia 26, 731 (1970).
Radler E.: The formation of nonvolatile acids by strains ofSaccharomyces during fermentation, p. 519 inYeasts. Models in Science and Technics (A. Kocková-Kratochvílová, E. Minárik, Eds). Publ. House Slovak Acad. Sci., Bratislava 1972.
Riemersma J.C.: Hydrogen ion transport during anaerobic fermentation by baker’s yeast. Thesis, Leiden 1964.
Rickard P.A.D., Hogan C.B.J.: Effect of glucose on the activity and synthesis of fermentative and respiratory enzymes ofSaccharomyces sp.Biotechnol.Bioeng. 20, 1105 (1978).
Rink H.: Regulatory function of H+—K+ exchange in yeast cells.Proc.IVth Internat.Symp.Yeasts, Part I, Abstr. A 33; Vienna 1975.
Sigler K., Knotková A., Kotyk A.: Effect of inhibitors on acid production by baker’s yeast.Folia Microbiol. 23, 410 (1978).
Slonimski P.P.: Adaptation respiratoire: dévelopment de la systéme hemoproteique induit par l’oxygene.Internat. Congr. Biochemistry 3, 242 (1956).
Sols A., Gancedo C, DelaFuente G.: Energy-yielding metabolism in yeasts, p. 271 inThe Yeasts (A.H. Rose, J.S. Harrison, Eds.). Vol. 2. Academic Press, London-New York 1971.
Watson T.G.: Effects of sodium chloride on steady-state growth and metabolism ofSaccharomyces cerevisiae.J.Gen.Microbiol. 64, 91 (1970).
Wurst M., Sigler K., Knotková A.: Gas chromatographic determination of extracellular metabolites produced by baker’s yeast during glucose-induced acidification.Folia Microbiol. 25, 000 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sigler, K., Knotková, A., Páca, J. et al. Extrusion of metabolites from baker’s yeast during glucose-induced acidification. Folia Microbiol 25, 311–317 (1980). https://doi.org/10.1007/BF02876611
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02876611