Abstract
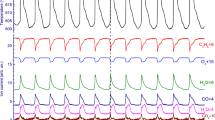
Kinetics of reaction between Na2S203 and peroxide compound (H202 or Na2S2O3) in a batch reactor and in a continuous stirring tank reactor (CSTR) were studied. Steady oscillations in uncatalyzed reactions in a CSTR were first discovered. In Na2S203-H2O2-H2S04 reaction system, Pt potential and pH of higher and lower flow rates beyond oscillation flow rates were in around the same extreme values. The reaction catalyzed by Cu2+ consists of the catalyzed oscillation process and the uncatalyzed osciliation one. On the basis of experiment, a reaction mechanism consisting of three stages was put forward. The three stages are H+ positive-feedback reactions, proton negative-feedback (uncatalyzed negative-feedback and catalyzed negative-feedback) reactions and transitional reactions. The mechanism is able to explain reasonably the nonlinear chemical phenomena appearing in the thiosulfate oxidation reaction by peroxide compounds.
Similar content being viewed by others
References
Orban, M., Epstein, I. R., The minimal permanganate oscillator and some derivates oscillator oxidation of S202- 3, SO2- 3, and S2- by pennanganate in a CSTR,J. Am, Chem. Soc., 1990, 112: 1812.
Rabai, G., Beck, M. T. Kenneth, K.et al., Sustained and damped pH oscillation in the perodate-thiosulfate reaction in a CSTR,J. Phys. Chem., 1989, 93: 2853.
Orban, M., Epstein, I. R., Chemical oscillators in group VIA: The Cu(II)-catalyzed reaction between hydrogen peroxide and Thiosulfateion,J. Am. Chem. Soc., 1987, 109: 101.
Orban, M., Epstein, I. R., Chemical oscillators in group VIA: The Cu(II)-catalyzed reaction between thiosulfate ion and peroxodisulfate ions,J. Am. Chem. Soc., 1989, 111: 2897.
Luo, Y., Epstein, I. R., A general model for pH oscillators,J. Am. Chem. Soc., 1990, 113: 1518.
Orban, M., Epstein, I. R., A new halogen-free chemical oscillator: The reaction between sulfide ion and hydrogen peroxide in a CSTR,J. Am. Chem. Sac., 1985, 107: 2302.
Gao Qingyu, Wang Yuemin, Zang Yaruet al., The nonlinear phenomena of the reactions between sulfur compound and hydrogen Peroxide,Acta Physico-chimica Sinicu (in Chinese), 1996, 12: 1.
Abel, E., Kinetics and catalysis of the reaction between hydrogen peroxide and thiosulfate,Monotcsh. Chem., 1907, 28: 1239.
Fava, A., Bresadola, S., Kinetics of the catalytic rearrangement of tetrathionate,J. Am. Chem. Soc., 1995, 77: 5792.
Naito, K., Shieh, M. C., Okabe, T., The chemical behavior of low valence sulfur compounds (V): Decomposition and oxidation of tetrathionate in aquous ammonia solution,Bulletin of the Chemical Society of Japan, 1970, 43: 1372.
Takizawa, M., Okuwaki, A., Okabe, T., The chemical behavior of low valence sulfur compounds ( VII ): The oxidation of sodium thiosulfate with ozone,Bulletin of the Chemical Society of Japan, 1973, 46: 3785.
Rabai, G., Orban, M., Epstein, I. R., Design of pH-regulated oscillators,Acc Chem. Res., 1990, 23: 258.
Luo, Y., Kustin, K., Epstein, I. R., Kinetics and mechanism of H202 decomposition catalyzed by cu2+ in alkaline solution,Inorg. Chem., 1988, 27: 2489.
Sillen, A. I., Martell, A. E.,Stability Constants of Metal-ion Complexes; Special Publication No.17, London: Chemical Society. 1964.
{au{fnRabai}, {gnG.}}, {au{fnEpstein}, {gnI. R.}}, {atEquilibria and kinetics of the fast interaction between copper(II) and thiosulfate ions in aqueous solution}, {jtInorg. Chem.}, {vn31} : {pp3239}.
Author information
Authors and Affiliations
Additional information
Project supported by the National Natural Science Foundation of China.
Rights and permissions
About this article
Cite this article
Gao, Q., Wang, Y., Wang, G. et al. Nonlinear chemical reaction between Na2S2O3 and Peroxide compound. Sc. China Ser. B-Chem. 40, 152–160 (1997). https://doi.org/10.1007/BF02876406
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02876406