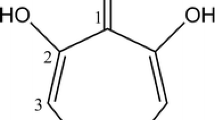
Abstract
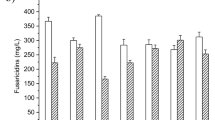
Late-exponential-phasePenicillium chrysogenum mycelia grown in a complex medium possessed an intracellular iron concentration of 650 μmol/L (2.2±0.6 μmol per g mycelial dry mass). This iron reserve was sufficient to ensure growth and antibiotic production after transferring mycelia into a defined low-iron minimal medium. Although the addition of Fe3+ to the Fe-limited cultures increased significantly the intracellular iron levels the surplus iron did not influence the production of penicillin V. Supplements of purified majorP. chrysogenum siderophores (coprogen and ferrichrome) into the fermentation media did not affect the β-lactam production and intracellular iron level. Neither 150 nor 300 μmol/L extracellular Fe3+ concentrations disturbed the glutathione metabolism of the fungus, and increased the oxidative stress caused by 700 mmol/L H2O2. Nevertheless, when iron was applied in the FeII oxidation state the oxidative cell injuries caused by the peroxide were significantly enhanced.
Similar content being viewed by others
Abbreviations
- DCF:
-
2′,7′-dichlorofluorescein
- GR:
-
glutathione reductase
- GSH:
-
glutathione
- GSSG:
-
glutathione disulfide
- IPNS:
-
isopenicillin N synthase
- PAA:
-
phenoxyacetic acid
References
Anderson M.E.: Determination of glutathione and glutathione disulphide in biological samples.Meth. Enzymol.113, 548–555 (1985).
Bainbridge Z.A., Scott R.I., Perry D.: Oxygen utilization by isopenicillin N synthase fromPenicillium chrysogenum.J. Chem. Tech. Biotechnol.55, 233–238 (1992).
Bundgaard H., Ilver K.: A new spectrophotometric method for the determination of penicillins.J. Pharm. Pharmac.24, 790–794 (1972).
Charlang G., Ng B., Horowitz N.H., Horowitz R.M.: Cellular and extracellular siderophores ofAspergillus nidulans andPenicillium chrysogenum.Mol. Cell Biol.1, 94–100 (1981).
Drechsel H., Winkelmann G.: Iron chelators and siderophores, pp. 1–49 in G. Winkelmann, C.J. Carrano (Eds):Transition Metals in Microbial Metabolism. Harwood Academic Publishers, Amsterdam 1997.
Dombovári J., Becker J.S., Kuhn A.J., Schröder W.H., Dietze H.J.: Multielement analysis of small plant tissue samples using inductively coupled plasma mass spectrometry.Atomic Spectr.21, 37–41 (2000a).
Dombovári J., Papp L., Mátyus J., Varga Z., Kakuk G.: Analysis of human blood, plasma and hair samples, using ICP-OES, GAAS and spectrographic methods.Magyar Kém. Folyóirat.106, 230–237 (2000b).
Emri T., Bartók G., Szentirmai A.: Regulation of specific activity of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase inPenicillium chrysogenum.FEMS Microbiol. Lett.117, 67–70 (1994).
Emri T., Pócsi I., Szentirmai A.: Phenoxyacetic acid induces glutathione-dependent detoxification and depletes the glutathione pool inPenicillium chrysogenum.J. Basic. Microbiol.37, 181–186 (1997a).
Emri T., Pócsi I., Szentirmai A.: Glutathione metabolism and the protection against oxidative stress caused by peroxides inPenicillium chrysogenum.Free Rad. Biol. Med.23, 809–814 (1997b).
Emri T., Pócsi I., Szentirmai A.: Changes in the glutathione (GSH) metabolism ofP. chrysogenum grown on different nitrogen, sulfur and carbon sources.J. Basic Microbiol.38, 3–8 (1998).
Emri T., Sámi L., Szentirmai A., Pócsi I.: Co-ordination of the nitrate and nitrite assimilation, the glutathione and free radical metabolisms, and the pentose phosphate pathway, inPenicillium chrysogenum.J. Basic Microbiol.39, 109–115 (1999a).
Emri T., Pócsi I., Szentirmai A.: Analysis of the oxidative stress response ofPenicillium chrysogenum to menadione.Free Rad. Res.30, 125–132 (1999b).
Emri T., Leiter É., Pócsi I.: Effect of phenoxyacetic acid on the glutathione metabolism ofPenicillium chrysogenum.J. Basic Microbiol.40, 93–104 (2000).
Eriksen S.H., Jensen B., Schneider I., Kaasgaard S., Olsen J.: Uptake of phenoxyacetic acid byPenicillium chrysogenum.Appl. Microbiol. Biotechnol.42, 945–950 (1995).
Halliwell B., Gutteridge J.M.C.:Free Radicals in Biology and Medicine. Oxford University Press, Oxford 1999.
van der Helm D., Winkelmann G.: Hydroxamates and polycarboxylates as iron transport agents (siderophores) in fungi, pp. 39–98 in G. Winkelmann, D.R. Winge (Eds):Metal, Ions in Fungi. Marcel Dekker, New York 1994.
Henriksen C.M., Nielsen J., Villadsen J.: Influence of the dissolved oxygen concentration on the penicillin biosynthetic pathway in steady-state cultures ofPenicillium chrysogenum.Biotechnol. Prog.13, 776–782 (1997).
Henriksen C.M., Nielsen J., Villadsen J.: Modelling of the protonophoric uncoupling by phenoxyacetic acid of the plasma membrane potential ofPenicillium chrysogenum.Biotechnol. Bioeng.60, 761–767 (1998).
Heymann P., Ernst J.F., Winkelmann G.: Identification of a fungal triacetylfusarinine C siderophore transport gene (TAF1) inSaccharomyces cerevisiae as a member of the major facilitator superfamily.BioMetals12, 301–306 (1999).
Hödt W., Römheld V., Winkelmann G.: Fusarinines and dimerum acid, mono- and dihydroxamate siderophores fromPenicillium chrysogenum, improve iron utilization by strategy I and strategy II plants.BioMetals13, 37–46 (2000).
Jaklitsch W.M., Hampel W., Röhr M., Kubicek C.P., Gamerith G.: α-Aminoadipate pool concentration and penicillin biosynthesis in strains ofPenicillium chrysogenum.Can. J. Microbiol.32, 473–480 (1986).
Jalal M.A.F., van der Helm D.: Isolation and spectroscopic identification of fungal siderophores, pp. 235–269 in G. Winkelmann (Ed.)CRC Handbook of Microbial Iron Chelators. CRC Press, Boca Raton 1991.
Jarvis F.G., Johnson M.J.: The mineral nutrition ofPenicillium chrysogenum Q176.J. Bacteriol.59, 51–60 (1950).
Ledenfeld T., Ghali D., Wolschek M., Kubicek-Pranz E.M., Kubicek C.P.: Subcellular compartmentation of penicillin biosynthesis inPenicillium chrysogenum.J. Biol. Chem.268, 665–671 (1993).
Lesuisse E., Labbe P.: Reductive iron assimilation inSaccharomyces cerevisiae, pp. 149–178 in G. Winkelmann, D.R. Winge (Eds):Metal Ions in Fungi. Marcel Dekker, New York 1994.
Nielsen J., Johansen C.L., Villadsen J.: Culture fluorescence measurements during batch and fed-batch cultivations withPenicillium chrysogenum.J. Biotechnol.38, 51–62 (1994).
Nielsen J.:Physiological Engineering Aspects of Penicillium chrysogenum. Polyteknisk Forlag, Lyngby (Denmark) 1995.
Peterson G.L.: Determination of total protein.Meth. Enzymol.91, 86–105 (1983).
Pinto M.C., Mata A.M., López-Barea J.: Reversible inactivation ofSaccharomyces cerevisiae glutathione reductase under reducing conditions.Arch. Biochem. Biophys.228, 1–12 (1984).
Pócsi I., Pusztahelyi T., Bogáti M.S., Szentirmai A.: The formation ofN-acetyl-β-d-hexosaminidase is repressed by glucose inPenicillium chrysogenum.J. Basic Microbiol.33, 259–267 (1993).
Pusztahelyi T., Pócsi I., Kozma J., Szentirmai A.: Aging ofPenicillium chrysogenum cultures under carbon starvation—I. Morphological changes and secondary metabolite production.Biotechnol. Appl. Biochem.25, 81–86 (1997).
Ramos F.R., López-Nieto M.J., Martín J.F.: Isopenicillin N synthase ofPenicillium chrysogenum, an enzyme that converts δ-(l-α-aminoadipyl)-l-cysteinyl-d-valine to isopenicillin N.Antimicrob. Agents Chemother.27, 380–387 (1985).
Sigler K., Chaloupka J., Brozmanová J., Stadler N., Höfer M.: Oxidative stress in microorganisms—I. Microbialvs. higher cells—damage and defenses in relation to cell aging and death.Folia Microbiol.44, 587–624 (1999).
White S., Berry D.R., McNeil B.: Effect of phenylacetic acid feeding on the process of cellular autolysis in submerged batch cultures ofPenicillium chrysogenum.J. Biotechnol.75, 173–185 (1999).
Wiebe C., Winkelmann G.: Kinetic studies on the specificity of chelate-iron uptake inAspergillus.J. Bacteriol.123, 837–842 (1975).
Winkelmann G.: Kinetics energetics, and mechanisms of siderophore iron transport in fungi, pp. 219–239 in L.L. Barton, B.C. Hemming (Eds):Iron Chelation in Plants and Soil Microorganisms. Academic Press, San Diego 1993.
Winkelmann G., Drechsel H.: Microbial siderophores, pp. 200–246 in H.-J. Rehn, G. Reed (Eds).Biotechnology, Vol. 7. VCH Press, Weinheim (Germany) 1997.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leiter, É., Emri, T., Gyémánt, G. et al. Penicillin V production byPenicillium chrysogenum in the presence of Fe3+ and in low-iron culture medium. Folia Microbiol 46, 127–132 (2001). https://doi.org/10.1007/BF02873590
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02873590