Abstract
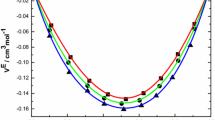
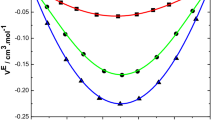
Excess molar volumes (VmE), viscosity deviations (Δlnη) and excess energies of activation for viscous flow (ΔG*E) are reported for non-electrolyte mixtures of 1,2-dimethoxyethane (monoglyme) and dichloromethane, trichloromethane, and tetrachloromethane at 298·15 K and at atmospheric pressure over the whole mole fraction range. The Prigogine-Flory-Patterson (PFP) model has been used to calculateVmE, and the results are compared with experimental data. The Bloomfield and Dewan model has been used to calculate viscosity coefficients, which are compared with experimental data for three mixtures. These results have been analysed in terms of dipole-dipole interactions between 1,2-dimethoxyethane and chloroalkanes. The magnitude of the strength of interaction decreases with the dipole character of the molecule.
Similar content being viewed by others
References
Pal A and Kumar A 1998Fluid Phase Equilibria 143 241
Pal A and Sharma S 1998J. Chem. Eng. Data 43 21
Pal A and Singh W 1996J. Chem. Thermodyn. 28 227
Pal A and Dass G 1999Indian J. Chem. Soc. A38 237
de Ruiz Holgado M E, de Schaefer C R, Arancibia E L and Katz M 1994Fluid Phase Equilibria 95 299
Inglese A 1992Thermochim. Acta 199 173
Spaneda A, Lepori L and Matteoli E 1991Fluid Phase Equilibria 69 209
Sharma S C, Joshi J M and Singh Y 1989J. Chem. Thermodyn. 21 331
Pal A and Singh W 1997Fluid Phase Equilibria 129 211
Pal A and Singh Y P 1996J. Chem. Eng. Data 41 1008
Douheret G, Davis M I, Hernandez M E and Flores H 1993J. Indian Chem. Soc. 70 395
Riddick J A, Bunger W B and Sakano T K 1986Techniques of chemistry 4th edn. (New York: Wiley-Interscience) vol. 2
Das B, Roy M N and Hazra D K 1994Indian J. Chem. Technol. 1 93
Dickinson E, Hunt D C and McLure I A 1975J. Chem. Thermodyn. 7 731
Pal A and Singh Y P 1994J. Chem. Thermodyn. 26 1063
IUPAC Commission on Atomic Weights and Isotopic Abundances 1985Pure Appl. Chem. 58 1677
Conclaves F A, Kestin J and Sengers J V 1991Int. J. Thermophys. 12 1013
Aucejo A, Cruz Burguet M, Munoz R and Sanchotello M 1996J. Chem. Eng. Data 41 508
Heric E I 1966J. Chem. Eng. Data 11 66
Patterson D and Delmas G 1970Discuss. Faraday Soc. 49 98
Prigogine I 1957The molecular theory of solution (Amsterdam: North Holland)
Flory P J 1965J. Am. Chem. Soc. 87 1833
Abe A and Flory P J 1965J. Am. Chem. Soc. 87 1838
Costas M and Patterson M 1982J. Sol. Chem. 11 807
Riedl B and Delmas G 1983Can. J. Chem. 61 1876
Reed T M III and Taylor T E 1959J. Phys. Chem. 63 58
Meyer R, Meyer M, Metzer J and Peneloux A 1971J. Chim. Phys. 62 405
Palepu R, Oliver J and Mackinnon B 1985Can. J. Chem. 63 1024
Bloomfield V A and Dewan R K 1971J. Phys. Chem. 75 3113
Celda B, Gavara R and Figueruelo J E 1987J. Chem. Eng. Data 32 31
Kanti M, Lagourette B, Alliez J and Boned C 1991Fluid Phase Equilibria 65 291
Tovar C A, Carballo E, Claudio A, Cerdeirina and Romani L 1997J. Chem. Eng. Data 42 1085
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pal, A., Dass, G. & Sharma, S. Excess molar volumes and viscosities of binary mixtures of 1,2-dimethoxyethane with chloroalkanes at 298·15 K. J Chem Sci 111, 659–668 (1999). https://doi.org/10.1007/BF02869121
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02869121