Abstract
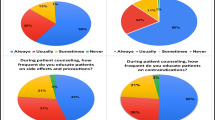
The Pharmacoepidemiologic Service of the Second University of Naples analyzed the use and tolerability of over-the-counter (OTC) oral nonsteroidal antiinflammatory drugs (NSAIDs) purchased in Campania, a region of southern Italy. Forty private pharmacies uniformly distributed throughout the region were recruited. The study was conducted by means of a questionnaire completed by purchasers and lasted from December 1, 1999 to March 31, 2000; 2053 questionnaires were collected. The age of respondents averaged 45.3 ± 3.49 years (range, 17–85 years). The NSAIDs analyzed were acetylsalicylic acid, paracetamol, ibuprofen, ketoprofen, diclofenac, and piroxicam. Adverse effects, mainly gastrointestinal symptoms, were reported by 5.5% of the users and occurred primarily with diclofenac, piroxicam, ibuprofen, and ketoprofen. Because the use and availability of OTC NSAIDs are increasing, further studies of the tolerability of this important drug class are warranted.
Similar content being viewed by others
References
Blenkinsopp A, Bradley C. Patients, society and the increase in self-medication.BMJ. 1996; 312:629–632.
Bradley C, Bond CM. Increasing the number of drugs available over-the-counter: arguments for and against.Br J Gen Pract. 1995;45:553–556.
Gossel TA. Implications of the reclassification of drugs from prescription-only to over-the-counter status.Clin Ther. 1991;13:200–215.
Ryan M, Yule B. Switching drugs for prescription-only to over-the-counter availability: economic benefit in the United Kingdom.Health Policy. 1990;16:233–239.
Smith MC. Prescription to OTC switches: reflections and projections.Drug Top. 1998;142:70–79.
Barber N. Drugs: from prescription only to pharmacy only.BMJ. 1993;307:640.
Schmid B. The safety assessment of over-the-counter products.Arch Toxicol. 1995;17:305–311.
Volans G. Monitoring the safety of over the counter drugs.BMJ. 1987;295:797–798.
Montastruc JL, Bagheri H, Geraud T. Pharmacovigilance of self-medication.Therapie. 1997; 52:105–110.
Levy M. Adverse reactions to over-the-counter analgesics: an epidemiologic evaluation.Agents Actions. 1988;25:21–31.
Garnett WR. GI effects of OTC analgesic: implications for product selection.J Am Pharm Assoc. 1996;NS36:565–572.
Blot WJ, McLaughlin JK. Over the counter non-steroidal anti-inflammatory drugs and risk of gastrointestinal bleeding.J Epidemiol Biostat. 2000;5:137–142.
Ceci A. OTC: situazione europea e realtá italiana. In:Atti del primo Forum Internazionale di Self-Medication. Intermedia: Brescia; 1996:51–58.
Sihvo S, Klaukka T, Martikainen J, Hemminki E. Frequency of daily over-the-counter drug use and potentially significant overprescription drug interactions in the Finnish adult population.Eur J Clin Pharmacol. 2000;56:495–499.
Nykamp D, Bernett CW, Hooper C. Risk of adverse drugs events related to ibuprofen use in a community sample.J Pharmacol Technol. 1994;10:110–114.
Honig PK, Gillespie BK. Drug interactions between prescribed and over-the-counter medication.Drug Saf. 1995;13:296–303.
Gaulg NJ, Shaw JP, Emmerton LM, Pethica BD. Surveillance of a recently switched non-prescription medicine (diclofenac) using a pharmacy-based approach.Pharmacoepidemiol Drug Saf. 2000; 9:207–214.
Bond CM, Bradley C. Over-the-counter drugs: the interface between the community pharmacist and patients.BMJ. 1996;312:758–760.
Gibson P, Henry D, Francis L. Association between availability of non-prescription beta-2-agonist inhalers and undertreatment of asthma.BMJ. 1993;306:1514–1518.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Motola, G., Russo, F., Mazzeo, F. et al. Over-the-counter oral nonsteroidal anti-inflammatory drugs: A pharmacoepidemiologic study in southern Italy. Adv Therapy 18, 216–222 (2001). https://doi.org/10.1007/BF02853167
Issue Date:
DOI: https://doi.org/10.1007/BF02853167