Abstract
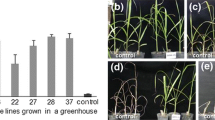
Transformed Russet Burbank and Lemhi Russet clones which contained three different transgene constructs were evaluated for performance under field conditions in Idaho. The transgenic lines were characterized over two growing seasons, using plants grown from both greenhouse produced minitubers (first year) and field grown seed (second year). Individual clones were evaluated for a variety of agronomic and quality properties. Many of the transformed clones showed reduced yield and increases in percent malformed and undersized tubers. Other characteristics, such as specific gravity and fry color, showed less variability. The variation observed in non-transgenic clones regenerated from tissue culture was less than that of the transformed lines. Out of an original population of 57 transgenic lines in tissue culture, maintenance of key agronomic and quality properties of the parental material was observed in only four clones. These results suggest that experiments designed to generate transgenic lines for the marketplace should be initiated with a large number of transgenic clones.
Compendio
Clones transformados de Russet Burbank y Lemhi Russet, que contenían tres diferentes prototipos de transgenes fueron evaluados para comportamiento bajo condiciones de campo en Idaho. Las líneas transgénicas se caracterizaron en dos temporadas de cultivo, utilizando plantas obtenidas tanto de minituberculos producidos en invernadero (primer año) como semilla producida en el campo (segundo año). Se evaluaron clones individuales para diversas propiedades agronomicas y de calidad. Muchos de los clones transformados mostraron rendimientos reducidos y un incremento en el porcentaje de tubérculos deformesy de pequeño tamaño. Otras características, tales como la gravedad específica y el color a la fritura, mostraron una menor variabilidad. La variacíon observada en los clones no transgénicos regenerados de cultivo de tejidos fue menor que aquella de las líneas transformadas. De una población original de 57 líneas transgénicas en cultivo de tejidos, se observó que sólo cuatro de ellas mantenían las principales propiedades agronómicas y de calidad del material progenitor. Estos resultados sugieren que los experimentos diseñados para generar líneas transgenicas para el mercado deberán iniciarse con un gran número de clones transgenicos.
Similar content being viewed by others
Literature Cited
An, G., B.D. Watson, S. Satchel, M.P. Gordon and E.W. Nester. 1985. New cloning vehicles for transformation of higher plants. EMBO J 4:277–284.
Bevan, M., R. Barker, A. Goldsbrough, M. Jarvis, T. Kavanagh and G. Iturriaga. 1986. The structure and transcription start site of a major tuber protein gene. Nucl Acids Res 14:4625–4638.
Bevan, M., W.M. Barnes and M.D. Chilton. 1983. Structure and transcription of the nopaline synthase gene region of T-DNA. Nucl Acids Res 11: 369–385.
Bourque, J.E., J.C. Miller and W.D. Park. 1987. Use of anin vitro tuberization system to study tuber protein gene expression. Cell Dev Biol 23: 381–386.
Dale, P.J. and H.C. MacPartlan. 1992. Field performance of transgenic potato plants compared with controls regenerated from tuber discs and shoot cuttings. Theor Appl Genet 84:585–591.
Destefano-Beltran, L., P.G. Nagpala, S.M. Cetiner, J.H. Dodds and J.M. Jaynes. 1990. Enhancing bacterial and fungal disease resistance in plants: application to potato.In: M.E. Vayda and W.D. Park (Eds). Molecular Biology of the Potato, Chapter 15, C.A.B. Internat Publ UK. pp. 89–95.
Dorel, C., T.A. Voelker, E.M. Herman and M.J. Chrispeels. 1989. Transport of proteins to the plant vacuole is not by bulk flow through the secretory system, and requires positive sorting information. J Cell Biol 108: 327–337.
Draper, J. and R. Scott. 1988. The isolation of plant nucleic acids.In: J. Draper, R. Scott, P. Armitage, and R. Waiden (Eds). Plant Genetic Transformation and Gene Expression: A Laboratory Manual. Blackstone Scientific Publications, Oxford. pp. 199–236.
Draper, J., R. Scott, and J. Hamil. 1988 Transformation of dicotyledonous plant cells using the Ti Plasmid ofAgrobacterium tumefaciens and the Ri plasmid of A.rhizogenes.In: Draper, R. Scott, P. Armitage and R. Waiden (Eds). Plant Genetic Transformation and Gene Expression: A Laboratory Manual. J. Blackstone Scientific Publications, Oxford. pp. 161–198.
Feldman, K.A. 1991. T-DNA insertion mutagenesis inAmbidopsis mutational spectrum. Plant J 1:71–82.
Hoekema, A., P.R. Hirsch, P.J.J. Hooykaas and R.A. Schilperoort. 1983. A binary plant vector strategy based on separation of the vir- and T-region ofAgrobacterium tumefaciens Ti plasmid. Nature 303:179–180.
Jefferson, R.A., T.A. Kavanagh and M.W. Bevan. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907.
Karp, A., M.J.K. Jones, D. Foulger, N. Fish and S.W.J. Bright. 1989. Variability in potato tissue culture. Am Potato J 66: 669–684.
Lawson, C., W. Kaniewski, L. Haley, R. Rozman, C. Newell, P. Sanders and N.E. Turner. 1990. Engineering resistance to mixed virus infection in a commercial potato cultivar: resistance to potato virus X and potato virus Yin transgenic Russet Burbank. Bio/Technol 8:127–134.
McBride, K.E. and K. Summerfelt. 1990. Improved binary vectors forAgrobacterium-mediated plant transformation. Plant Mol Biol 14:269–276.
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15: 473–497.
Odell, J.T., F. Nagy F and N. H. Chua. 1987. Variability in 35S promoter expression between independent transformants.In: JL Key and L McIntosh (Eds). Plant Gene Systems and Their Biology. Alan R. Liss, New York, NY, pp 321–330.
Ottaviani, M-P., T. Smits and C.H. Hanisch ten Cate. 1993. Differential methylation and expression of the β-glucuronidase and neomycin phosphotransferase genes in transgenic plants of potato cv, Bintje. Plant Sci 88: 73–81
Pavek, J.J., D.L. Corsini, J.G. Gamer, S. Michener, W.C. Sparks, G.F.Cranahan, C.E. Stanger, A.R. Mosley, M.J. Johnson, G.E. Carter, R.E. Voss, M.W. Martin and R.H. Johansen. 1981. Lemhi Russet: a new high yielding potato variety with wide adaptation, attractive tubers, and high internal quality. Am Potato J 58: 619–625.
Ray, A., N.A. Memmel, R.P. Orchekowski and K.A. Kumaran. 1987. Isolation of two cDNA clones coding for larval hemolymph proteins ofGalleria mellonella. Insect Biochem 17:603–617.
Rickey, T.M. and W.R. Belknap. 1991. Comparison of the expression of severals tress-responsive genes in potato tubers. Plant Mol Biol 16: 1009–1018.
Sambrook, J., E.F. Fritsch, T. Maniatis: Molecular Cloning: A Laboratory Manual (2nd ed). 1989. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
Rietveld, R.C., P.M. Hasegawa and R.A. Bressan. 1991. Somaclonal variation in tuber discderived populations of potato. I. Evidence of genetic stability across tuber generations and diverse locations. Theor Appl Genet 82: 430–440.
Secor, G.A. and J.F. Shephard. 1981. Variability of protoplast derived potato clones. Crop Sci 21: 102–105.
Snyder, G.W. and W.R. Belknap. 1993. An improved method for Agrobacterium-mediated transformation ofin vitro-grown potato microtubers. Plant Cell Reports. In Press.
Tefler, W.H., S.P. Keim and J.H. Law. 1983. Arylphorin, a new protein fromHyalophora cecropia. comparisons with calliphorin and manducin. Insect Biochem 13: 601–613.
Vayda, M.E. and W.R. Belknap. 1992. The Emergence of transgenic potatoes as commercial products and tools for basic science. Transgenic Research 1: 149–163.
Verwoerd, T.C., B.M.M. Dekker and A. Hoekema. 1989. A small-scale procedure for the rapid isolation of plant RNAs. Nucl Acids Res 17: 2362.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Belknap, W.R., Corsini, D., Pavek, J.J. et al. Field performance of transgenic Russet Burbank and Lemhi Russet potatoes. American Potato Journal 71, 285–296 (1994). https://doi.org/10.1007/BF02849055
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02849055