Abstract
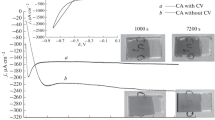
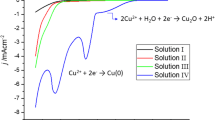
Photocurrent characteristics of the Cu2O/Pt and Cu2O/TiO2 photoelectro-chemical cells have been presented. In aqueous solution, a slow deterioration of power output occurs approximately at the rate of 50% per day. Operation in non-aqueous solutions (acetonitrile and ethanol) also produced a deterioration of power output. However in ethanol, it was found that the deterioration reversed itself and a new cycle of deterioration and rejuvenation began. These suggest that the deterioration is not due to a chemical reduction process at the electrode surface but other factors such as migration of charged defect centres in the depletion layer or chemical reaction on the electrode surface.
Similar content being viewed by others
References
Khan K A, Leung Y K, Kos J F and Koffyberg F P 1981Proc. SESCI Conference, University of Quebec, CanadaD17
Kohl P A and Brad A J 1979J. Electrochem. Soc. 126 598
Memming R 1978Phillips Tech. Rev. 38 160
Nagasubramanian G, Gioda A S and Brad A J 1981J. Electrochem. Soc. 128 1258
Nozik A J 1978Annu. Rev. Chem. 29 189
Pfound A H 1916Phys. Rev. 7 289
Scaife D E 1980Solar Energy 25 41
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Khan, K.A., Kos, J.F. Photocurrent characteristics in Cu2O/Pt and Cu2O/TiO2 photoelectrochemical cells in aqueous and non-aqueous electrolytes. Pramana - J. Phys. 26, 277–281 (1986). https://doi.org/10.1007/BF02845268
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02845268