Abstract
Development of recombinant DNA vaccine against hepatitis B grown on cultured yeast cell has made it possible to mount a world-wide effort to control and eradicate Hepatitis B infection. However, the currently recommended schedules (0, 1 & 2 months, and 0–1 and 6 months) do not coincide with the scheduled visits for other E.P.I. vaccines, and necessitate additional visits for Hepatitis B vaccination. This study was therefore carried out to find out if adequate seroconversion occurs to Hepatitis B vaccine when given with other EPI vaccines or not?
Thirty nine infants born to Australia antigen positive mothers from among 850 screened pregnant mothers were recruited to receive Hepatitis B vaccine (Engerix B-10 micro gram each) at 0, 6 and 14 wks (group A) or at 0, 1 and 2 months (group B). Thirty-one infants were recruited in group A and 8 in group B. The cord blood was collected and the first dose of vaccine was given within 48 hours of birth. Simultaneous B.C.G. was given at the left deltoid. Other E.P.I. vaccines were given qt 6, 10 and 14 wks in group A and at 2, 3 and 4 months in group B. Repeat blood samples were collected prior to giving each dose of Hepatitis B vaccine, and 4 weeks after the last dose. All blood samples were assayed for HBsAg and HBsAb at the National Institute Of Communicable Diseases, utilizing standard ELISA kits.
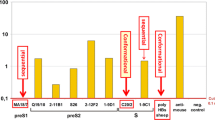
The seroconversion rates following one, two and three doses of Hepatitis B vaccine were 3.33%, 55.5%, 96.15% and 0%, 62.5% and 100% in group A and B respectively. Only one infant in group A failed to develop HbsAb even after 3 doses of Hepatitis B vaccine, 5 infants in group A were available at 9 months of age and were still positive for HBsAb and negative for HBsAg. Recombinant DNA Hepatitis B vaccine (Engerix-B) is a highly effective vaccine which can safely and effectively be given together with other childhood vaccines.
Similar content being viewed by others
References
Ghendon Y. WHO strategy for the global elimination of new cases of Hepatitis B.Vaccine 1990; 8 (Suppl): 129–132.
Hoofnagle JH. Towards universal immunisation against Hepatitis B virus.N Engl J Med 1989; 321: 1333–1334.
Maynard JE, Hepatitis B: Global importance and need for control.Vaccine 1990; 8 (Suppl. 1): 18–20.
Sobeslavsky O. Prevalence of markers of Hepatitis B virus infection in various countries: A WHO collaborative study.Bull WHO 1980; 58: 621–628.
Sung JL. Hepatitis B virus eradication strategy for Asia.Vaccine 1990; 8 (Suppl): 95–98.
West DJ, Calandra GB, Ellis RW. Vaccination of infants and children against Hepatitis B.Pediatr Clin North Am 1990; 37: 585–603.
Yeoh EK. Hepatitis B virus infection in children.Vaccine 1990; 8 (Suppl): 29–30.
Andre FE, Safary A. Summary of clinical findings on Engerix B, a genetically engineered yeast derived Hepatitis B vaccine.Post Grad Med J 1987; 63 (2): 169–178.
Centres for Diseases Control. Update on Hepatitis B prevention. Recommendations of the immunization practices advisory committee.Ann Intern Med 1987; 107: 353–357.
Maynard JE, Kane MA, Hadler SC. Global control of Hepatitis B through vaccination—Role of Hepatitis B vaccine in EPI.Rev Infect Dis 1989; 11 (Suppl 3): 574–578.
Expanded Programme on Immunization Cost-effectiveness.Weekly Epidemiology Record 1982; 22: 170–172.
Guha DK, Mahajan J, Aggarwal SK et al. Vertical transmission of Hepatitis B virus.Indian Pediatr 1988; 25: 409–416.
Gupta I, Sehgal A, Bhakoo DN et al. Immunoprophylaxis of neonates born to Hepatitis B surface antigen positive pregnant women. Proceedings “Update in Viral Hepatitis” 1990; 137–143.
Gupta ML, Sharma U, Saxena S et al. Vertical transmission of Hepatitis B. Surface antigen from asymptomatic carrier mothers.Indian Pediatr 1985; 22: 339–342.
Kane MA, Hadler SC, Marglis HS et al. Routine prenatal screening for Hepatitis B surface antigen.JAMA 1988; 259: 408–409.
Bortolotti F, Cadrobbi P, Crivellaro et al. Long term outcome of chronic type B Hepatitis in patients who acquire Hepatitis B virus infection in childhood.Gastroenterology 1990; 99: 805–810.
Cadranel S, Zeghlache S, Fernandes S et al. Vaccination of newborns of HBsAg positive carrier mothers with a recombinant DNA Hepatitis B caccine.Post Grad Med J 1987; 63 (Suppl 2): 159–160.
Hollinger FB, Troisi CL, Pepe PE. Anti HBs responses to vaccination with a human Hepatitis B vaccine made by recombinant DNA technology in yeast.J Infect Dis 1986; 153: 156–159.
Memorandum from a WHO meeting. Progress in control of viral Hepatitis.Bull WHO 1988; 66: 443–455.
Piazza M, Villari R, Orlando R et al. Hepatitis B immunisation with a reduced number of doses in newborn babies and children.Lancet 1985; ii: 949–952.
Zachoval R, Jilg W, Lorbeer B et al. Passive-active immunisation against Hepatitis B.J Infect Dis 1984; 150: 112–117.
Coursaget P, Relyveld EH, Barres JL et al. Simultaneous administration of Hepatitis B vaccine and DPT-polio vaccine.Infection and Immunity 1986; 51: 784–787.
Chiron JP, Coursaget P, Yvomet B et al. Simultaneous administration of Hepatitis B and diphtheria, tetanus, polio vaccine.Lancet 1984; 1: 623–624.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mittal, S.K., Rao, S., Kumari, S. et al. Simultaneous administration of Hepatitis B vaccine with other E.P.I. vaccines. Indian J Pediatr 61, 183–188 (1994). https://doi.org/10.1007/BF02843614
Issue Date:
DOI: https://doi.org/10.1007/BF02843614