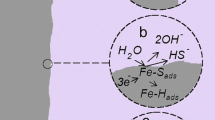
Abstract
Iron oxide development is necessary as the Iron electrodes exhibit high self discharge and poor charging efficiency in alkaline batteries. Pressed electrodes containing electrolytic iron powder with varying amounts of Fe3O4 have been used. The variation of open circuit potential and self discharge currents with alkali concentration is followed. For better understanding of these variations, cyclic polarisation (−1.3 V to + 0.4 Vvs Hg/HgO) and hydrogen evolution studies are carried out. Beyond −0.5 Vvs Hg/HgO, the surface is covered by hydrolysed layer and the protons diffuse away from this layer. The hydrogen evolution takes place with the discharge of K+ ions as the rate determining step.
Similar content being viewed by others
References
Ahmed S M 1969J. Phys. Chem. 73 3546
Ahmed S M 1972The anodic behaviour of metals and Semi Conductors ed. Diggle Vol.1, p 463
Blok L and de Bruyn P L 1970J. Colloid. Interface Sci. 32 518, 527
Burke L D and Lyons M E G 1986J. Electroanal. Chem. Interfacial Electrochem 198 347
Cerny J and Micka K 1989J. Power Sources 25 111
Dibrov I A, Chervyak Voronish S M, Grigoreva T V and Kozlova G M 1980Electrokhimiya 16 786
Falk S V and Salkind A J 1969Alkaline Storage batteries (John Wiley & Sons Inc. NY) 94
Flerov V N, Uzinger L V and Pavlova L I 1964Zh. Prikl. Khim 37 373
Flerov V N, Pavlova L I and Uzinger L V 1965Zh. Prikl. Khim 38 569
Galushko V P, Zavagorodnaya E F and Galvoronskaya L K 1960Zh. Prikl. Khim 33 1546
Galushko V P, Zavagorodnyaya E F and Afanasenko V I 1961Zh. Prikl. Khim 34 1271
Labat J, Jarousseau J C and Laurent J F 1970Power sources 3 Proc. 7th International Symposium, Brighton, 283
Lishanski L M, Fantgof V M and Efremov B N 1982Electrokhimiya 18 644
Micka K and Zabransky Z 1987J. Power Sources 19 315
Muralidharan V S and Rajagopalan K S 1978J. Electronal Interfacial Electrochem 94 21
Muralidharan V S and Veerashanmugamani M 1985J. Appl. Electrochem. 15 675
Muralidharan V S 1988B. Electrochem 4 651
Paruthimal Kalaignan G, Muralidharan V S and Vasu K I 1985Proceedings of the annual technical meeting of Electrochemical Society, 32
Paruthimal Kalaignan G, Muralidharan V S and Vasu K I 1987J. Appl. Electrochem 17 1083
Sato N 1989Corrosion 45 354
Schindler P W, Walti B and Furst B 1976Chimica 30 107
Sigg L and Stumn W 1980/81Colloids Surf 2 101
Teplinskaya T K, Fedorova N N and Rozenstsveig S A 1964Zh. Fiz. Khim 38 2167
Thangavel K, Muralidharan V S and Rajagopalan K S 1982,J. Electrochem Soc. (India)31 49
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jayalakshmi, M., Muralidharan, V.S. An insight into the self-discharge of Fe3O4 electrodes in alkali solutions. Proc. Indian Acad. Sci. (Chem. Sci.) 103, 161–171 (1991). https://doi.org/10.1007/BF02843566
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02843566