Abstract
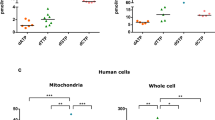
A specific diaphorase was defective in cultured skin fibroblasts from a patient with Luft disease (giant mitochondrial disease; nonthyroid hypermetabolism). In homogenates of whole cells, diaphorase activity was 124±8 nmol/min/mg protein, compared to 425±40 in 14 controls. On fractionation of the cells with digitonin, the whole decrease was attributable to a decrease in the soluble fraction (57±11 vs 597±73 nmol/min/mg protein). The decrease persisted with NADPH as substrate as well as NADH, and with several electron acceptors. On electrophoresis and activity staining, the Luft cells lacked the major, cathodic, FAD-dependent protein with diaphorase activity. The pathophysiological significance of the deficiency of this diaphorase has not been studied, but the defect might impair the regulation of metabolism by altering the control of cellular redox state.
Similar content being viewed by others
References
Afifi A. K., Ibrahim M. Z. M., Bergman R. A., Haydar N. A., Mire J., Bahuth N., and Kaylani F. (1972) Morphologic features of hypermetabolic mitochondrial disease. A light-microscopic histochemical, and electron-microscopic study.J. Neurol. Sci. 15, 271–290.
Atkin B. M., Utter M. F., and Weinberg M. B. (1979) Pyruvate carboxylase and phosphoenolpyruvate carboxylkinase activity in leukocytes and fibroblasts from a patient with pyruvate carboxylase deficiency.Pediatr. Res. 13, 38–43.
Bearn A. G. (1976) The expression of inherited metabolic disease in cultured cells.Harvey Lect. 70, 75–91.
Blass J. P. (1982) Inborn errors of pyruvate metabolism, inMetabolic Basis of Inherited Disease 5th ed. (Stanbury J. B., Wyngaarden J., Fredrickson D. S., Goldstein J., Brown M., eds.) pp. 193–203. McGraw-Hill, New York, NY.
Blass, J. P. and Gibson G. E. (1979) Consequences of mild, graded hypoxia.Adv. Neurol. 26, 229–250.
Blass J. P., Milne J. A., and Rodnight R. (1977) Newer concepts of psychiatric diagnosis and biochemical research on mental illness.Lancet 1, 738–740.
Breakfield X. O., Casiglione C. M., and Edelstein S. B. (1976) Monoamine oxidase activity decreased in cells lacking hypoxanthine phosphoribosyltransferase activity.Science 192, 1018–1020.
DiMauro S., Bonilla E., Reed C. P., Schotland D. L., Scarpa A., Conn H., Jr., and Chance B. (1976) Luft’s Disease—Further biochemical and ultrastructural studies of skeletal muscle in the second case.J. Neurol. Sci. 27, 217–232.
Edwards Y. H., Potter J., and Hopkinson D. A. (1980) Human FAD-dependent NAD(P)H diaphorase.Biochem. J. 187, 429–436.
Grzeschik, K. H. (1980) Assignment of a structural gene for a fourth human diaphorase (DIA4) to chromosome 16 in man-mouse somatic cell hybrids.Hum. Genet. 53, 189–193.
Haydar N. A., Conn H. L., Afifi A., Wakid N., Ballas S., and Fawaz K. (1971) Severe hypermetabolism with primary abnormality of skeletal muscle mitochondria.Ann. Intern. Med. 74, 548–558.
Hulquist D. E. and Passon P. G. (1971) Catalysis of methaemoglobin reduction of erythrocyte cytochrome b5 and cytochrome b5 reductase.Nature (New Biology) 229, 252–254.
Johnson W. G. (1981) The clinical spectrum of hexosaminidase deficiency diseases.Neurology 31, 1453–1456.
Kaplan J.-C., and Beutler E. (1967) Electrophoresis of red cell NADH- and NADPH-dipahorases in normal subjects and patients with congenital methemoglobinemia.Biochem. Biophys. Res. Commun. 29, 605–610.
Kark R. A. P., Brown W. J., Edgerton V. R., Reynolds S. F., and Gibson G. (1975) Experimental thiamine deficiency. Neuropathic and mitochondrial changes induced in rat muscle.Arch. Neurol. 32, 818–825.
Lowry O. H., Rosebrough N. J., Farr A. L., and Randall R. J. (1951) Protein measurements with the Folin phenol reagent.J. Biol. Chem. 193, 265–275.
Luft R., Ikkos D., Palmieri G., Ernster L., and Afzelius B. (1962) A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical, and morphological study.J. Clin. Invest. 41, 1776–1804.
Mitchell P. (1979) Keilin’s respiratory chain concept and its chemiosmotic consequences.Science 206, 1148–1159.
Piacentini-Sorbi S., Sheu K. F. R., and Blass J. P. (1982a) An enzymatic defect in giant mitochondrial (Luft’s disease) fibroblasts.Clin. Res. 30, 293.
Piacentini-Sorbi S., Sheu K. F. R., and Blass J. P. (1982b) Subcellular distribution of diaphorase and its deficiency in giant mitochondrial disease.Fed. Proc. 41, 531.
Reeves W. J. and Fimognari G. M. (1966) (l)-Lactic dehydrogenase: Heart (H4), inMethods in Enzymology, Volume IX, (Wood W. A., ed.) pp. 288–294. Academic Press, New York.
Scott E. M. and Griffith I. V. (1959) The enzyme defect of hereditary methemoglobinemia: diaphorase.Biochim. Biophys. Acta 34, 584–586.
Stumpf D. A. and Parks J. K. (1979) Friedreich ataxia. II. Normal kinetics of lipoamide dehydrogenase.Neurology 29, 820–826.
Veeger G. and Massey V. (1962) The reaction mechanism of lipoamide dehydrogenase. II. Modification of trace metals.Biochim. Biophys. Acta 64, 83–100.
Wallin R. (1979) Some molecular properties of NAD(P)H dehydrogenase from rat liver.Biochem. J. 181, 127–135.
West C. A. (1972) A study of the inheritance and properties of an electrophoretic variant of NADH-methaemoglobin reductase associated with congenital methaemoglobinaemia.Br. J. Haematol. 23, 343–349.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Piacentini-Sorbi, S., Sheu, KF.R. & Blass, J.P. Deficiency of a specific diaphorase in Luft disease fibroblasts. Neurochemical Pathology 1, 147–157 (1983). https://doi.org/10.1007/BF02834195
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02834195