Abstract
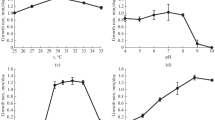
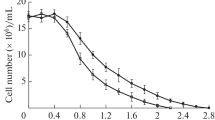
Effects of four lipid peroxidation-inducing pro-oxidants-amphiphilictert-butyl hydroperoxide (TBHP), hydrophobic 1,1′-azobis(4-cyclohexanecarbonitrile) (ACHN), hydrophilic Fe11 and 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH)-on cell growth and on generation of peroxidation products in isolated plasma membrane lipids were determined in four yeast species (S. cerevisiae, S. pombe, R. glutinis andC. albicans) differing in their plasma membrane lipid composition. TBHP and ACHN inhibited cell growth most strongly, Fe11 and AAPH exerted inhibitory action for about 2 h, with subsequent cell growth resumption.S. cerevisiae strain SP4 was doped during growth with unsaturated linoleic (18∶2) and linolenic (18∶3) acids to change its resistance to lipid peroxidation. Its plasma membranes then contained some 30% of these acids as compared with some 1.3% of 18∶2 acid found in undopedS. cerevisiae, while the content of (16∶1) and (18∶1) acids was lower than in undopedS. cerevisiae. The presence of linoleic and linolenic acids inS. cerevisiae cells lowered cell survival and increased the sensitivity to pro-oxidants. Peroxidationgenerated conjugated dienes (CD) were measured in pure TBHP- and ACHN-exposed fatty acids used as standards. The CD level depended on the extent of unsaturation and the pro-oxidant used. The TBHP-induced CD production in a mixture of oleic acid and its ester was somewhat lower than in free acid and ester alone. In lipids isolated from the yeast plasma membranes, the CD production was time-dependent and decreased after a 5–15-min pro-oxidant exposure. ACHN was less active than TBHP. The most oxidizable were lipids fromS. cerevisiae plasma membranes doped with linoleic and linolenic acids and fromC. albicans with indigenous linolenic acid.
Similar content being viewed by others
Abbreviations
- AAPH:
-
2,2′-azobis(2-amidinopropane)dihydrochloride
- ACHN:
-
1,1′-azobis(4-cyclohexanecarbonitrile)
- CD:
-
conjugated dienes
- FAME:
-
fatty acid methyl esters
- PUFA:
-
oligounsaturated fatty acids
- TBHP:
-
tert-butyl hydroperoxide
- TBRS:
-
thiobarbituric acid-reactive substances
- YEPD:
-
yeast extract-peptone-dextrose
References
Allen C.F., Good P.: Acyl lipids in photosynthetic systems.Meth. Enzymol.123, 523–547 (1971)
Biliński T., Śledziewski A., Rytka J.: Hemoprotein formation in yeast—VI. Mutants with changed levels of catalase and of other heme enzymes under conditions of glucose repression.Acta Microbiol. Polon.29, 183–186 (1980).
Bisson L., Coons D.M., Kruckeberg A.L., Lewis D.A.: Yeast sugar transporters.Crit. Rev. Biochem. Mol. Biol.28, 259–308 (1993).
Bjornstedt M., Hamberg M., Kumar S., Xue J., Holmgren A.: Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocysteine strongly stimulates the reactionvia catalytically generated selenolsJ. Biol. Chem.270, 11761–11764 (1995).
Bossie M.A., Martin C.E.: Nutritional regulation of yeast Δ-9 fatty acid desaturase activity.J. Bacteriol.171, 6409–6413 (1989).
Brown D.A., London E.: Structure and origin of ordered lipid domains in biological membranes.J. Membr. Biol.164, 103–114 (1998).
Dufour J.P., Amory A., Goffeau A.: Plasma membrane ATPase from the yeastSchizosaccharomyces pombe.Meth. Enzymol.157, 513–528 (1988).
Evans M.V., Tunton H.E., Grant C.M., Dawies J.W.: Toxicity of linoleic acid hydroperoxide toSaccharomyces cerevisiae: involvement of a respiration-related process for maximal sensitivity and adaptive response.J. Bacteriol.180, 483–490 (1998).
Gruszecki W.J.: Carotenoids in membranes, pp. 363–379 in H.A. Frank, A.J. Young, G. Britton, R.J. Coydell (Eds):The Photochemistry of Carotenoids. Kluywer Academy Publishers, Dordrecht (The Netherlands) 1999.
Halliwel B., Gutterige J.M.C.:Free Radicals in Biology and Medicine, 3rd ed., pp. 284–313. Oxford University Press, Oxford (UK) 1999.
Howlett N.G., Avery S.V.: Induction of lipid peroxidation during heavy metal stress inSaccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation.Appl. Environ. Microbiol.63, 2971–2976 (1997).
Krasowska A., Lukaszewicz M., Oswiecimska M., Witek S., Sigler K.: Spontaneous and radical-induced plasma membrane lipid peroxidation in differently oxidant-sensitive yeast species and its suppression by antioxidants.Folia Microbiol.45, 509–514 (2000).
Krasowska A., Stasiuk M., Oswiecimska M., Kozubek A., Bien M., Witek S., Sigler K.: Suppression of radical-induced lipid peroxidation in a model system by alkyl esters of cinnamate quaternary ammonium salts.Z. Naturforsch.C56, 878–885 (2001).
Marinho H.S., Antunes F., Pinto R.E.: Role of glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase in the reduction of lysophospholipid hydroperoxides.Free Rad. Biol. Med.22, 871–883 (1997).
McElhaney R.N.: The biological significance of alterations in the fatty acid composition of microbial membrane lipids in response to changes in environmental temperature, pp. 176–189 in M.R. Heinrich (Ed.).Extreme Environments—Mechanisms of Microbial Adaptation. Academic Press, New York 1976.
Prescha A., Swiędrych A., Biernat J., Szopa J.: Increase in lipid content in potato tubers modified by 14-3-3 gene overexpression.J. Agric. Food Chem.49, 3638–3643 (2001).
Pryor W.A., Castle L.: Chemical methods for the detection of lipid hydroperoxides.Meth. Enzymol.105, 293–297 (1984).
Serrano R.: Transport across yeast vacuolar and plasma membrane, pp. 523–585 in E.W. Jones, J.R. Broach (Eds).The Molecular and Cellular Biology of the Yeast SaccharomycesGenome Dynamics, Protein Synthesis and Energetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY) 1991.
Sigler K., Chaloupka J., Brozmanová J., Stadler N., Höfer M.: Oxidative stress in microorganisms-I. Microbialversus higher cells—damage and defenses in relation to cell aging and death.Folia Microbiol.44, 587–624 (1999).
Singh B., Oberoi G.K., Sharma S.C.: Effect of pH stress on lipid composition ofSaccharomyces cerevisiae.Indian J. Exp. Biol.28, 430–433 (1990).
Subczyński W.K., Markowska E., Siewiesiuk J.: Effect of polar carotenoids on the oxygen diffusion-concentration product in lipid bilayers. An ESR spin label study.Biochim. Biophys. Acta1068, 68–72 (1991).
Subczyński_W.K., Wiśnicka A.: Physical properties of lipid bilayer membranes: relevance to membrane biological functions.Acta Biochim. Polon.47, 613–626 (2000)
Subczyński W.K., Hyde J.S., Kusumi A.: Effect of alkyl chain unsaturation and cholesterol intercalation on oxygen transport in membranes: a pulse ESR spin labeling study.Biochemistry30, 8578–8590 (1991).
Suutari M., Liukkonen K., Laakso S.: Temperature adaptation in yeast: the role of fatty acids.J. Gen. Microbiol.136, 1469–1474 (1990).
Tsuchihashi H., Kigoshi M., Iwatsuki M., Niki E.: Action of β-carotene as an antioxidant against lipid peroxidation.Arch. Biochem. Biophys.323, 137–147 (1995).
Wiśniewska A., Subczyński W.K.: Effect of polar carotenoids on the shape of the hydrophobic barrier of phospholipid bilayers.Biochim. Biophys. Acta1368, 235–246 (1998).
Yin J.J., Subczyński W.K.: Effects of lutein and cholesterol on alkyl chain bending in lipid bilayers: a pulse electron spin resonance spin labeling study.Biophys. J.71, 832–839 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krasowska, A., Chmielewska, L., Prescha, A. et al. Viability and formation of conjugated dienes in plasma membrane lipids ofSaccharomyces cerevisiae, schizosaccharomyces pombe, rhodotorula glutinis andCandida albicans exposed to hydrophilic, amphiphilic and hydrophobic pro-oxidants. Folia Microbiol 47, 145–151 (2002). https://doi.org/10.1007/BF02817672
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02817672