Abstract
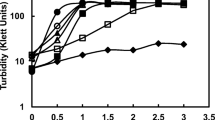
SixBradyrhizobium (lupin) strains were evaluated for their ability to produce siderophores using four chemical assays. Two strains gave positive reactions with chrome azurol S assay (CAS) and produced hydroxamate-type siderophores. The other four strains gave negative results for siderophore production using the four assays. Generation time, growth yield and hydroxamate production of one strain (WPBS 3201 D) were affected by the iron concentration of the culture medium and the previous culture history of the cells. Resuspension of washed cells grown previously in media supplemented with 0 and 20 μmol/L Fe into differing iron regimes (0, 0.5, 1, 2, 4, 8, 10, 15 and 20 μmol/L Fe) suggest that the extent of hydroxamate production depended on the growth history of the cells. Cells pregrown in 20 μmol/L Fe produced a high amount of hydroxamates compared with cells pregrown in iron-free medium when resuspended in medium containing up to 4 μmol/L Fe. Cells pregrown in 20 μmol/L Fe were more sensitive to iron repression than those pregrown in 0.5 μmol/L Fe. Mannitol was the best carbon source for siderophore production. Siderophore synthesis was inhibited by 4-chloromercuribenzenesulfonic acid, 2,4-dinitrophenol, sodium azide and MgCl2 suggesting that an energized membrane and a mercapto group are essential and required for hydroxamate synthesis in strain WPB5 3201 D.
Similar content being viewed by others
References
Alexander B.D., Zuberer D.A.: Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria.Biol. Fert. Soils12, 39–45 (1991).
Brown C.M., Dilworth M.J.: Ammonia assimilation byRhizobium cultures and bacteroids.J. Gen. Microbiol.86, 39–48 (1975).
Carrillo-Castaneda G., Peralta J.R.V.: Siderophore-like activies inRhizobium phaseoli.J. Plant Nutr.11, 935–944 (1988).
Carson K.C., Dilworth M.J., Glenn A.R.: Siderophore production and iron transport inRhizobium leguminosarum bv.viciae MNF710.J. Plant. Nutr.15, 2203–2220 (1992).
Crowley D.E., Reid C.C.P., Szanislo P.J.: Microbial siderophores as iron sources for plants, pp. 375–386 in G. Winkelman, D. Van der Helm, J.B. Neilands (Eds):Iron Transport in Microbes, Plant and Animals. VCH Publishers, New York 1987.
Eady R.R., Postgate J.R.: Nitrogenase.Nature249, 805–819 (1974).
Francis A.J., Alexander M.: Catalase activity and nitrogen fixation in legume root nodules.Can. J. Microbiol.18, 861–864 (1972).
Gibson F., Margrath D.I.: The isolation and characterization of hydroxamic acid (aerobactin) formed byAerobacter aerogenes 62-1.Biochim. Biophys. Acta192, 175–184 (1969).
Guerinot M.L.: Iron uptake and metabolism in the rhizobia/legume symbioses.Plant & Soil130, 199–209 (1991).
Guerinot M.L., Meidl E.J., Plessner O.: Citrate as a siderophore inBradyrhizobium japonicum.J. Bacteriol.172, 3298–3303 (1990).
Hemantraranjan A., Garg O.K.: Introduction of nitrogen fixing nodules throught iron and zinc fertilization in the non nodule-forming fernch bean (Phaseolus vulgaris L.).J. Plant Nutr.9, 281–288 (1986).
Modi M., Shah K.S., Modi V.V.: Isolation and characterization of catechol-like siderophore from cowpeaRhizobium RA-1.Arch. Microbiol.141, 156–158 (1985).
Mollering H., Gruber W.: Determination of citrate with citrate lyase.Anal. Biochem.17, 369–376 (1966).
Neilands J.B.: Microbial envelope proteins related to iron.Ann. Rev. Microbiol.36, 285–309 (1982).
O'Hara G.W., Dilworth M.J., Bookerd N., Parkpian P.: Iron deficiency specifically limits nodule development in peanut inoculated withBradyrhizobium sp.New Phytol.108, 51–57 (1988).
Rai R., Singh S.N., Prasad V.: Effect of presumed amended pyrite on symbiotic N2-fixation, active iron content of nodules, grain yield and quality of chickpea (Cicer arietinumLinn.) genotypes in calcareous soil.J. Plant Nutr.5, 905–913 (1982).
Reeves M., Pine L., Neilands J.B., Ballows A.: Absence of siderophore activity inLegionella sp. grown in iron deficient media.J. Bacteriol.154, 324–329 (1983).
Rioux C.R., Jordan D.C., Rattary J.B.M.: Iron requirement ofRhizobium leguminosarum and secration of anthranilic acid during growth on iron-deficient medium.Arch. Biochem. Biophys.248, 175–182 (1986).
Smith M.J., Neilands J.B.: Rhizobactin, a siderophore fromRhizobium meliloti.J. Plant Nutr.7, 449–458 (1984).
Tang C., Robson A.D., Dilworth M.J.: The role of iron in nodulation and nitrogen fixation inLupinus angustifolius L.New Phytol.114, 173–182 (1990).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abd-Alla, M.H. Growth and siderophore production inBradyrhizobium (lupin) strains under iron limitation. Folia Microbiol 44, 196–200 (1999). https://doi.org/10.1007/BF02816242
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02816242