Abstract
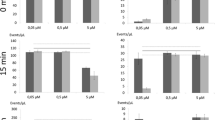
Three fluorescent probes, tetramethyl rhodamine ethyl ester (TMRE), 3,3′-dipropylthiacarbocyanine iodide (diS-C3(3)) and 3,3′-dipropyloxacarbocyanine iodide (diO-C3(3)), were tested for their suitability as fluorescent indicators of membrane potential inSaccharomyces cerevisiœ in studies performed by flow cytometry. For all these dyes the intensity of fluorescence of stained cells increased with probe concentration in the range of 60–3000 nmol/L. The optimum staining period was 15–20 min for diS-C3(3). Depolarization of cells by increased extracellular potassium level and by valinomycin elicited with all probes a drop in fluorescence intensity. In some yeast batches this depolarization was accompanied by a separation of subpopulations with different fluorescence properties.
Similar content being viewed by others
References
Kurzweilová H., Sigler K.: Fluorescent staning with bromocresol purple: A rapid method for determining yeast cell dead count developed as an assay of killer toxin activity.Yeast 9, 1207–1211 (1993).
Kurzweilová H., Sigler K.: Comparison of three different methods for determining yeast killer toxin K1 activity and standardisation of units.Experientia 51, 26–29 (1995).
Loew L.M.: Membrane potential imaging, pp. 255–272 in R.J. Cherry (Ed.):New Techniques of Optical Microscopy and Microspectroscopy. Macmillan Press, London 1991.
Loew L.M.: Potentiometric membrane dyes, pp. 150–160 in W.T. Mason (Ed.):Fluorescent and Luminiscent Probes for Biological Activity. Academic Press, San Diego 1993.
Midgley M., Thompson C.L.: The role of mitochondria in the uptake of methyltriphenylphosphonium ion bySaccharomyces cerevisiœ.FEMS Microbiol. Lett. 26, 311–315 (1985).
van de Mortel J.B.J., Mulders D., Korthout H., Theuvenet A.P.R., Borst-Pauwels G.W.F.H.: Transient hyperpolarization of yeast by glucose and ethanol.Biochim. Biophys. Acta 936, 421–428 (1988).
Plášek J., Dale R.E., Sigler K., Laskay G.: Transmembrane potentials in cells: A diS-C3(3) assay for relative potentials as an indicator of real changes.Biochim. Biophys. Acta 1196, 181–190 (1994).
Plášek J., Denksteinová B., Surreau F.: Assessment of membrane potential using confocal microspectrofluorimetry.J. Fluorescence 3, 157–159 (1993).
Plášek J., Sigler K.: Slow fluorescent indicators of membrane potential: a survey of different approaches to probe response analysis.J. Photochem. Photobiol. B: Biol. 33, 101–124 (1996).
Shapiro H.M.: Cell membrane potential analysis, pp. 121–133 in D.L. Taylor, Y. Wang (Eds):Methods Cell Biology, Vol. 41. Academic Press, San Diego 1994.
Smith J.C.: Potential-sensitive molecular probes in membranes of bioenergetic relevance.Biochim. Biophys. Acta 1016, 1–28 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Denksteinová, B., Sigler, K. & Plášek, J. Three fluorescent probes for the flow-cytometric assessment of membrane potential inSaccharomyces cerevisiœ . Folia Microbiol 41, 237–242 (1996). https://doi.org/10.1007/BF02814623
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02814623