Abstract
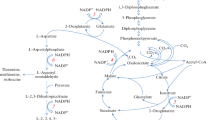
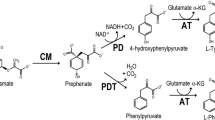
A number of tryptophan-plus-tyrosine double auxotrophs have been isolated from a glutamate producingArthrobacter globiformis excretingl-phenylalanine by two-step mutagenesis with N-methyl-N′-nitro-N-nitrosoguanidine. For the three potent mutants tested the medium of Alföldi was found to be the best. The optimum tryptophan, tyrosine and biotin concentrations for phenylalanine production of these mutants were 0.5 mmol/L, 0.1 mmol/L and 5 μg/L, respectively. At these levels strain TT-39 yielded 2.6 g phenylalanine per L of medium in flask culture with glucose (350 mmol/L) and NH4Cl (60 mmol/L).
Similar content being viewed by others
References
Adelberg E.A.: Selection of bacterial mutants which excrete antagonists of antimetabolites.J. Bacteriol. 76, 326–326 (1958).
Adelberg E.A., Morton M., Grace Chem C.C.: Optimal conditions for mutagenesis by N-methyl-N′-nitro-N-nitrosoguanidine inE. coli K12.Biochem. Biophys. Res. Commun. 18, 788–795 (1965).
Alföldi L.: La production induite de megacine en milieu synthetique.Ann. Inst. Pasteur 94, 474–484 (1958).
Akashi K., Shibai H., Hirose Y.: Effect of O2 supply onl-phenylalanine,l-proline,l-glutamine andl-arginine fermentation.J. Ferment. Technol. 57, 321–327 (1979).
Brown K.D., Roy C.H.: Control of aromatic amino acid biosynthesis: the multiplicity of 7-phospho-2-oxo-3-deoxyd-arabino-heptonated-erythrose 4-phosphate lyase (pyruvate phosphorylating) inE. coli W.Biochim. Biophys. Acta 104, 377–389 (1965).
Coats J.H., Nester E.W.: Regulation reversal mutation: characterisation of end product-activated mutants ofBacillus subtilis.J. Biol. Chem. 242, 4948–4955 (1967).
Calhown D.H., Jensen R.A.: Significance of altered carbon flow in aromatic amino acid synthesis: an approach to the isolation of regulatory mutants inPseudomonas aeruginosa.J. Bacteriol. 109, 365–372 (1972).
Choi Y.J.: Phenylalanine production byE. coli. A. feasibility study.PhD Thesis. University of New South Wales 1981.
Choi Y.J., Tribe D.E.: Continuous production of phenylalanine using anE. coli regulatory mutant.Biotechnol. Lett. 4, 223–228 (1982).
Cotton R.G.H., Gibson F.: The biosynthesis of phenylalanine and tyrosine enzyme converting chorismic acid into prephenic acid and their relationships to prephenate dehydratase and prephenate dehydrogenase.Biochim. Biophys. Acta 100, 76–88 (1965).
Davis B.D.: Studies on nutritionally deficient bacterial mutant isolated by means of penicillin.Experientia 6, 41–50 (1950).
Davis B.D., Mingioli E.S.: Mutants ofE. coli requiring methionine or vitamin B12.J. Bacteriol. 60, 17–28 (1950).
Hagino H., Nakayama K.:l-Phenylalanine production by analog-resistant mutants ofCorynebacterium glutamicum.Agr. Biol. Chem. 38, 157–161 (1974a).
Hagino H., Nakayama K.: DAHP synthetase and its control inCorynebacterium glutamicum.Agr. Biol. Chem. 38, 2125–2134 (1974b).
Hagino H., Nakayama K.: Regulatory properties of prephenate dehydrogenase and prephenate dehydratase fromCorynebacterium glutamicum.Agr. Biol. Chem. 38, 2367–2376 (1974c).
Hagino H., Nakayama K.: Regulatory properties of chorismate mutase fromCorynebacterium glutamicum.Agr. Biol. Chem. 39, 331–342 (1975).
Huang H.T.: Production ofl-phenylalanine byE. coli.Appl. Microbiol. 9, 419–423 (1961).
Hwang S.O., Gil G.H., Cho Y.J., Kang K.R., Lee J.H., Bee J.C.: The fermentation process forl-phenylalanine production using an auxotrophic regulatory mutant ofE. coli.Appl Microbiol. Biotechnol. 22, 108–113 (1985).
Jensen R.A., Nasser D.S., Nester E.W.: Comparative control of a branch point enzyme in microorganisms.J. Bacteriol. 94, 1582–1593 (1967).
Kinoshita S.: The production of amino acids by fermentation process.Adv. Appl. Microbiol. 1, 201–214 (1959).
Kinoshita S., Udaka S., Shimono M.: Studies on the amino acid fermentation. I. Production ofl-glutamic acid by various microorganisms.J. Gen. Appl. Microbiol. (Tokyo) 3, 193–205 (1957).
Klausner A.: Building for success in phenylalanine.Bio/Technology 3, 301–307 (1985).
Kim S.W., Pittard J.: Phenylalanine biosynthesis inE. coli: mutants derepressed for chorismate mutase,P-prephenate-dehydratase.J. Bacteriol. 106, 784–790 (1971).
Koch G.L.E., Shaw D.C., Gibson F.: The purification and characterization of chorismate mutase, prephenate dehydrogenase fromE. coli B12.Biochim. Biophys. Acta 229, 795–804 (1971).
Lederberg J., Lederberg E.M.: Replica plating and indirect selection of bacterial mutants.J. Bacteriol. 63, 399–406 (1952).
Nakayama K., Kitada S., Sato Z., Kinoshita S.J.: Induction of nutritional mutants of glutamic acid bacteria and their amino acid accumulation.J. Gen. Appl. Microbiol. (Tokyo) 7, 41–51 (1961a).
Nakayama K., Sato Z., Kinoshita S.: Production ofl-phenylalanine byCorynebacterium glutamicum.Nippon Nogei-Kagaku Kaishi 35, 142–147 (1961b).
Nester E.W., Jensen R.A.: Control of aromatic acid biosynthesis inBacillus subtilis: sequential feedback inhibition.J. Bacteriol. 91, 1595–1598 (1966).
Okumura S., Otsuka S., Yamanoi A., Yoshinaga F., Honda T., Kubota K., Tsuchida T. l-Phenylalanine.US Pat. 3 600 235 (1972).
Otsuka S., Miyajima R., Shiio I.: Comparative studies on the mechanism of microbial glutamate fermentation from glucose inBrevibacterium flavum andMicrococcus glutamicus.J. Gen. Appl. Microbiol. (Tokyo) 11, 295–301 (1965)
Polsinelli M.: Production ofl-phenylalanine byp-fluorophenylalanine resistant mutant ofBacillus subtilis.Giorn. Microbiol. 13, 99–104 (1965).
Roy D.K., Chatterjee S.P.: Production of glutamic acid by anArthrobacter sp. I. Nutritional requirement in relation to glutamic acid production.Acta Microbiol. Polon. 3, 117–122 (1982).
Robinson D.S.: Oxidation of selected alkanes and related compounds by aPseudomonas strain.Antonie van Leeuwenhoek 30, 303–316 (1964).
Shetty K., Crawford D.L., Pometto IIIA.L.: Production ofl-phenylalanine from starch by analog-resistant mutants ofBacillus polymyxa.Appl. Environ. Microbiol. 52, 637–643 (1986).
Suzuki M., Berglund A., Unden A., Heden C.G.: Aromatic amino acids production by analog-resistant mutants ofMethylomonas methanolophila 6R.J. Ferment. Technol. 55, 466–475 (1977).
Shiio I., Otsuka S., Takahashi M.: Effects of biotin on the bacterial formation of glutamic acid. I. Glutamate formation and cellular permeability of amino acids.J. Biochem. (Tokyo) 51, 56–62 (1962).
Smith L.C., Ravel J.M., Lax S.R., Shieve W.: The control of 3-deoxy-d-arabino-heptulosonic acid 7-phosphate synthesis by phenylalanine and tyrosine.J. Biol. Chem. 237, 3566–3570 (1962).
Sugimoto S., Miyajima R., Tsuchida T., Shiio I.: Regulation of aromatic acid biosynthesis and production of tyrosine and phenylalanine inBrevibacterium flavum.Agr. Biol. Chem. 37, 2327–2336 (1973).
Tokoro Y., Oshima K., Okii M., Yamaguchi K., Tanaka K., Kinoshita S.: Microbial production ofl-phenylalanine fromn-alkanes.Agr. Biol. Chem. 34, 1516–1521 (1970).
Tsuchida T., Kubota K., Morinaga Y., Matsui H., Enei H., Yoshinaga F.: Production ofl-phenylalanine by a mutant ofBrevibacterium lactofermentum 2256.Agr. Biol. Chem. 51, 2095–2101 (1987).
Tokoro Y., Oshima K., Tanaka K., Kinoshita S.: Production of amino acids from hydrocarbon.Amino Acids Nucl Acids 19, 115–119 (1969).
Tanaka K., Iwasaki H., Kinoshita S.: Glutamic acid fermentation. V. Biotin andl-glutamic acid accumulation by bacteria.Nippon Nogei Kagaku Kaishi 34, 593–599 (1960a).
Tanaka K., Akita S., Kimura K., Kinoshita S.: Glutamic acid fermentation. VI. The role of biotin in the metabolism ofM. glutamicus.Nippon Nogei Kagaku Kaishi 34, 600–608 (1960b).
Udaka S., Kinoshita S.: Development of mutants with relaxed regulatory mechanism for amino acid production.J. Gen. Appl. Microbiol. (Tokyo) 4, 283–291 (1958).
Veldkamp H., Berg Z., Zevenhuizen L.P.T.M.: Glutamic acid production byArthrobacter globiformis.Antonie van Leeuwenhoek J. Microbiol. Serol. 29, 35–51 (1963).
Wallance B.J., Pittard J.: Genetic and biochemical analysis of the isozymes concerned in the first reaction of aromatic biosynthesis inE. coli.J. Bacteriol. 93, 237–244 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maiti, T.K., Chatterjee, S.P. l-Phenylalanine production by double auxotrophic mutants ofArthrobacter globiformis . Folia Microbiol 36, 234–239 (1991). https://doi.org/10.1007/BF02814354
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02814354