Abstract
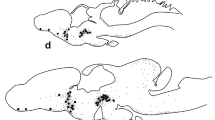
Antiserum against testis ecdysiotropin isolated from the gypsy moth,Lymantria dispar, reacted with neurons in the protocerebrum, optic and antennal lobes, subesophageal, thoracic and abdominal ganglia, as well as in nerve tracts extending through the optic lobes, tritocerebrum, and interganglionic connectives of the pupal stage of these insects. Testis ecdysiotropin is a peptide required by immature moths to initiate production of testes ecdysteroid, which is necessary for the development of the male reproductive system and initiation of spermatogenesis.
Antiserum against testis ecdysiotropin also detected an accumulation of testis ecdysiotripic-like material between the inner and outer testis sheaths of pupae. The localization of this peptide in the imaginal disks of the last larval stage, cells and nerve fibers in the optic and antennal lobes of the pupa of both sexes, as well as in the testes during development of the adult reproductive system indicates that testis ecdysiotropin has a much larger impact on adult metamorphosis than development of the reproductive system and initiation of gametogenesis. Although this peptide may have a modulatory role in the central nervous system (CNS), it may also initiate a cascade of activity required for the development of the adult nervous system, in addition to its role in reproduction.
Similar content being viewed by others
References
Bell R. A., Owens C. D., Shapiro M, and Tardif J. R. (1981) Mass rearing and virus production, inThe Gypsy Moth: Research Toward Integrated Pest Management (Doane, C. C. and McManus M. L., eds.), USDA, Washington, DC, pp. 599–655.
Blomquist G. J. and Dillwith J. W. (1983) Pheromones: biochemistry and physiology, inEndocrinology of Insects (Downer R. G. H. and Laufer H., eds.), Liss, New York, pp. 527–542.
Bollenbacker W. E. and Bowen M. F. (1983) The prothoracicotropic hormone, inEndocrinology of Insects (Downer R. G. H. and Laufer H., eds), Liss, New York, pp. 89–99.
Bollenbacher W. E., Agui N., Granger N., and Gilbert L. I. (1980) Insect prothoracic glands in vitro: a system for studying the prothoracicotropic hormone, inInvertebrate Systems in Vitro (Kurstak E., Maramorosch K. K., and Dubendorfer A., eds.), Elsevier/North Holland Biomedical Press, Amsterdam, Holland, pp. 253–271.
Copenhaven P. F. and Truman J. W. (1986) Metamorphosis of the cerebral neuroendocrine system in the moth.Manduca sexta. J. Comp. Neur. 249, 186–204.
Girardie J., Huet R. O., Nespoulos C., Van Dorsselaer A., and Pernollet J. C. (1991) Physical characterization and sequence identification of the ovary maturating parsin, a new neurohormone purified from the nervous corpora cardiaca of the african locust (Locusta migratoria).Eur. J. Biochem. 202, 1121–1126.
Giebultowicz J. M., Riemann J. G., Raina A. K., and Ridgeway R. L. (1989) Circadian system controlling release of sperm in insect testes.Science 245, 1098–1100.
Gillot C. and Ismail P. M. (1995) In vitro synthesis of ecdysteroid by the male accessory reproductive glands, testis and abdominal integument of the adult migratory grasshopper,Melanoplus sanguinipes.Invertebr. Reprod. Dev. 27, 65–71.
Girardie J. and Girardie A. (1977) Liberation provoquee in vitro dup produit de neuro secretion des cellules protocerebralis medianes chez le criquet migrateur.J. Physiol. (Paris) 73, 707–721.
Gray R. S., Muehleisen D. P., and Bollenbacher W. E. (1994) Multiple peptide expression by the L-NSC in the tobacco hornworm,Manduca sexta, inInsect Neurochemistry and Neurophysiology 1993 (Borkovec A. B. and Loeb M. J., eds.), CRC, Boca Raton, FL pp. 251–254.
Hagadorn H. H., O'Connor J. D., Fuchs M. S., Sage B., Schlaeger D. A., and Bohm M. K. (1975) Ovary as a source of α-ecdysone in an adult mosquito.Proc. Natl. Acad. Sci. USA 72, 3255–3259.
Hoffmann K. H., Weidner K., and Seidel M. (1992) Sites of ecdysteroid biosynthesis in female adults ofGryllus bimaculatus.J. Comp. Physiol. B 162, 731–739.
Homberg U., Davis N. T., Hildebrand J. G. (1990) Peptide-immunocytochemistry of neurosecretory cells in the brain and retrocerebral complex of the sphinx moth, Manduca sexta.J. Comp. Neurol. 303, 35–52.
Loeb M. J. (1986) Ecdysteroids in testis sheaths ofHeliothis virescens larvae: an immunocytochemical study.Arch. Insect Biochem. Physiol. 3, 173–180.
Loeb M. J. (1991) Growth and development of sperm-ducts of the tobacco bud worm moth,Heliothis virescens, in vivo and in vitro.Invertebr. Reprod. Dev. 19, 97–105.
Loeb M. J. and Hakim R. S. (1991) Development of genital imaginal discs ofHeliothis virescens culture in vitro with 20-hyroxyocdysone and fat body or testis sheaths.Invertebr. Reprod. Devel. 20, 181–191.
Loeb M. J., Woods C. W., Brandt E. P., and Borkovec A. B. (1982) Larval testes of the tobacco budworm: a new source of insect ecdysteroids.Science 26, 896–897.
Loeb M. J., Brandt E. P., Woods C. W., and Borkovec A. B. (1987) An ecdysiotropic factor from brains ofHeliothis virescens induces testes to produce immunodetectable ecdysteroid in vitro.J. Exp. Zool. 243, 275–282.
Loeb M. J., Brandt E. P., Woods C. W., and Bell R. A. (1988) Secretion of ecdysteroid by sheaths of testes of the gypsy moth,Lymantria dispar, and its regulation by testis ecdysiotropin.J. Exp. Zool. 248, 94–100.
Loeb M. J., Kochansky J., Wagner R. M., and Bell R. A. (1994) Transduction of the signal initiated by the neuropeptide, testis ecdysiotropin, in testes of the gypsy moth,Lymantria dispar.J. Insect Physiol. 40, 939–946.
Loeb, M. J., Wagner R. M., Woods C. W., Gelman D. G., Harrison D., and Bell R. A. (1997) Naturally occurring analogs ofLymantria testis ecdysiotropin, a gonadotropin isolated from brains ofLymantria dispar pupae.Arch. Insect Biochem. Physiol. 36, 37–50.
Meola S. M. and Loeb M. J. (1995) Unique intertesticular tissue complex in larvae ofHeliothis virescens (F.) (Lepidoptera: Noctuidae).Int. J. Insect MorphoL Embryol. 24, 443–457.
Meola S. M., Clottens F. L., Coast G. M., and Holman G. M. (1994) Localization of leucokinin VIII in the cockroach,Leucophaea maderae, using an antiserum directed against an achetakinin-I analog.Neurochem. Res. 19, 805–814.
Nassel D. R., Shiga S., Mohrherr J., and Rao K. R. (1993) Pigment-dispersing hormone-like peptide in the nervous system of the fliesPhormia andDrosophila: Immunocytochemistry and parial characterization.J. Comp. Neurol. 331, 183–198.
Nordlander R. H. and Edwards J. S. (1968) Morphology of the larval and adult brains of the monarch butterfly,Danaus plexippus, L.J. Morphol. 126, 67–94.
O'Brien M. A., Katahira E. J., Flanagan T. R., Arnold L. W., Haughton G., and Bollenbacher W. E. (1988) A monoclonal antibody to the insect prothoracicotropic hormone.J. Neurosci. 8, 3247–3257.
Pierantoni R. (1974) An observation on the giant fibre posterior optic tract in the fly.Biokybernetik (Leipzig) 5, 157–163.
Plotnikova S. A. (1969) Affector neurones with several axons in the ventral cord ofLocusta migtatoria.J. Evol. Biochem. Physiol. 5, 339–341.
Reimann J. G., Thorson B. J., and Ruud R. L. (1974) Daily cycle of release of sperm from the testes of the mediterranean flour moth.J. Insect Physiol. 20, 195–207.
Shimizu T., Moribayashi A., and Agui N. (1985) In vivo analysis of spermiogenesis and testicular ecdysteroids in the cabbage armyworm,Mamestra brassicae L.Appl. Entomol. Zool.,20, 56–61.
Truman J. W. and Copenhaver P. F. (1989) The larval eclosion hormone neurones inManduca sexta: identification of the brain-proctodeal neurosecretory system.J. Exp. Biol. 47, 457–470.
Truman J. W., Hewes R. S., and Ewer J. (1994) Action and interaction of peptides in regulating ecdysis behavior in insects, inInsect Neurochemistry and Neurophysiology 1993 (Borkovec A. B. and Loeb M. J., eds.), CRC, Boca Raton, FL, pp.39–51.
Wagner R. M., Loeb M. J., Kocheensky J. P., Gelman D. B., Lusby W. R., and Bell R. A. (1997) Identification and characterization of an ecdysiotropic peptide from brain extracts of the gypsy moth,Lymantria dispar.Arch. Insect Biochem. Physiol. 34, 175–189.
Weevers R. de G. (1985) The insect ganglia, inComprehensive Insect Physiology Biochemistry and Pharmacology (Kerkut G. A. and Gilbert L. L., eds.), Pergamon, Oxford, UK, pp. 213–297.
Westbrook A. L., Regan S. L., and Bollenbacher W. E. (1993) Developmental expression of the prothoracicotropic hormone in the CNS of the tobacco hornwormManduca sexta.J. Comp. Neurol. 327, 1–16.
Zitnan D., Kingan T. G., Kramer S. J., and Beckage N. (1995) Accumulation of Neuropeptides in the cerebral neurosecretory system ofManduca sexta larvae parasitized by the braconid waspCotesia congregata.J. Comp. Neurol. 356, 83–100.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meola, S.M., Loeb, M., Kochansky, J.P. et al. Immunocytochemical localization of testis ecdysiotropin in the pupa of the gypsy moth,Lymantria dispar (L.) (Lepidoptera: Lymantriidae). J Mol Neurosci 9, 197–210 (1997). https://doi.org/10.1007/BF02800502
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02800502