Abstract
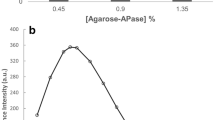
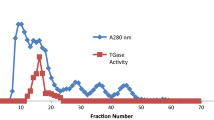
Pig muscle aldolase was insolubilized by covalent attachment to a polyacrylamide matrix containing carboxylic functional groups. The catalytic activity of the Akrilex C-aldolase was 2014 units/g solid, i.e., an activity loss of only about 5% relative to the initial activity. The pH optimum for catalytic activity shifted form 7.25 to 7.5 and the apparent temperature optimum from 313 to 318 K. The Michaelis constant of the insolubilized enzyme was significantly higher than that of the soluble aldolase. Heat- and urea-inactivation experiments revealed that the immobilization increased the stability of the enzyme.
Similar content being viewed by others
References
Brown, H. D., Patel, A. B., and Chattopadhyay, S. K. (1968),J. Chromatog.35, 103.
Bernfeld, P., and Wang, J. (1963),Science 142, 678.
Marshall, D. J. (1974), inImmobilized Biochemicals and Affinity Chromatography, Dunlap, R. B., ed., Plenum, New York, pp. 354–356.
Bernfeld, P., Bieber, R. E., and MacDonnel, P. C. (1968),Arch. Biochem. Biophys. 127, 779.
Chan, W. W.-C., and Mawer, H. M. (1972),Arch. Biochem. Biophys. 149, 136.
Falb, R. D., Linn, J., and Shapira, J. (1973),Experientia 29, 958.
Schell, H. D., Scholnic, L., and Carstenau, M. (1977),Stud. Cercet. Biochim. 20, 77.
Pollak, A., Blumenfeld, H., Wax, M., Baughn, R. L., and Whitesides G. M. (1980),J. Am. Chem. Soc. 102, 6324.
Ábrahám, M., Horváth, L., and Szajáni, B. (1985), Comp. Biochem. Physiol, in press.
Szajáni, B., Ivony, K., and Boross, L. (1980),Acta Biochim. Biophys. Acad. Sci. Hung. 15, 295.
Szajáni, B., Ivony, K., and Boross, L. (1980),J. Appl. Biochem. 2, 72.
Kovács, K., Szajáni, B., and Boross, L. (1980),J. Appl. Biochem. 4, 11.
Lowry, O. H., Rosebrough, N. J., Farr, L., and Randall, R. J. (1951),J. Biol. Chem. 193, 503.
Sibley, J. A., and Lehninger, A. L. (1948),J. Biol. Chem. 177, 859.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ábrahám, M., Horvth, L., Simon, M. et al. Characterization and comparison of soluble and immobilized pig muscle aldolases. Appl Biochem Biotechnol 11, 91–100 (1985). https://doi.org/10.1007/BF02798541
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02798541