Abstract
The kinetics of immobilized pig heart fumarase are described and compared with the properties of the enzyme free in solution.
-
1.
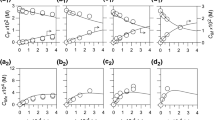
An analogous pH dependence of initial activity is found for free and immobilized enzyme.
-
2.
Immobilized and free fumarase deviate from classical Michaelis-Menten kinetics in the same way. The apparent Km values are three to eight times higher for the immobilized (2 mg/g gel) enzyme.
-
3.
The specific activity of immobilized fumarase is dependent on the final enzyme concentration on the gel; normal specific activities are observed when 50 ⧎g fumarase is immobilized per gram of gel, whereas the specific activity decreases with increasing enzyme concentration.
-
4.
The activation energies for free and immobilized fumarase (50 ⧎g/g gel) were found to be identical between 22 and 32‡C and with L-malate as substrate (Ea = 12,290 cal/mol at pH 7.9). Upon increasing the concentration of fumarase on the gel, the activation energy decreases.
Our results indicate that the true catalytic properties of fumarase are not affected by immobilization of this enzyme. The slight differences observed when fumarase is immobilized at concentrations higher than 50 ⧎g/g gel must be attributed to diffusional limitation at the surface of the Sepharose matrix.
Similar content being viewed by others
References
Beeckmans, S., and Kanarek, L. (1981),Eur. J. Biochem. 117, 527.
Müller, J., and Pfleiderer, G. (1980),Hoppe-Seyler’s Z. Physiol. Chem. 361, 675.
Erekin, N., and Friedman, M. E. (1979),J. Solid-Phase Biochem. 4, 123.
Kanarek, L., and Hill, R. L. (1964),J. Biol. Chem. 239, 4202.
Cuatrecasas, P. (1970),J. Biol. Chem. 245, 3059.
Alberty, R. A., Massey, V., Frieden, C., and Fuhlbrigge, A. R. (1954),J. Am. Chem. Soc. 76, 2485.
Hill, R. L., and Kanarek, L. (1964),Brookhaven Symp. Biol. 17, 80.
Fasman, G. D. (1963),Meth. Enzymol. 6, 928.
Alberty, R. A., and Bock, R. M. (1953),Proc. Nat. Acad. Sci. USA 39, 895.
Beeckmans, S., Vandenbranden, S., and Kanarek, L. (1980),Arch. Intern. Physiol. Bioch. 88, B187.
Frieden, C., and Alberty, R. A. (1955),J. Biol. Chem. 212, 859.
Massey, V. (1953),Biochem. J. 53, 72.
Lilly, M. D., Hornby, W. E., and Crook, E. M. (1966),Biochem. J. 100, 718.
Hornby, W. E., Lilly, M. D., and Crook, E. M. (1968),Biochem. J. 107, 669.
Kay, G., and Lilly, M. D. (1970),Biochim. Biophys. Acta 198, 276.
Sundaram, P. V., and Crook, E. M. (1971),Can. J. Biochem. 49, 1388.
Royer, G. P., and Green, G. M. (1971),Biochem. Biophys. Res. Comm. 44, 426.
Julliard, J. H., Godinot, C., and Gautherion, C. (1971),FEBS Lett. 14, 185.
Mukherjee, A., and Srere, P. A. (1978),J. Solid-Phase Biochem. 3, 85.
Sundaram, P. V., and Joy, K. W. (1978),J. Solid-Phase Biochem. 3, 223.
Sundaram, P. V. (1978),J. Solid-Phase Biochem. 3, 241.
Engasser, J-M. (1978),Biochim. Biophys. Acta 526, 301.
Affinity Chromatography: Principles and methods, edited by Pharmacia Fine Chemicals, 1979, p. 17.
Lehninger, A. L. (1978),Biochemistry, 2nd ed., Worth, New York, p. 783.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Beeckmans, S., Kanarek, L. Kinetics of immobilized pig heart fumarase. Appl Biochem Biotechnol 7, 177–188 (1982). https://doi.org/10.1007/BF02798295
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02798295