Abstract
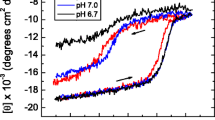
The stability of invertase was studied under various conditions, including at 75°C, in presence of stabilizers (sorbitol and glycerol) at 75°C, and in the presence of denaturants (urea and trichloroacetic acid) at 37°C in reverse micelles. Stability of the invertase in reverse micelles was found to be improved over that of the enzyme in bulk aqueous solution. Sorbitol could enhance enzyme stability as it does in the bulk aqueous system. The stabilizing effect of glycerol was reduced in reverse micelles. The denaturation pattern of urea remains unaltered. However, the denaturation effect of trichloroacetic acid has been reduced in reverse micelles.
Similar content being viewed by others
References
Ulbrich-Hofmann, R. and Selisko, B. (1993),Enzyme Microb. Technol. 15, 33–41.
Madamwar, D. B., Bhatt, J. P., and Ray, R. M. (1988),Enzyme Microb. Technol. 10, 302–305.
Hochkoeppler, A. and Palmieri, S. (1990),Biotechnol. Bioeng. 36, 672–677.
Hagen, A. J., Hatton, T. A., and Wang, D. I. C. (1990),Biotechnol. Bioeng. 35, 955–965.
Luisi, P., Meyer, P., and Wolf, R. (1980),Enzyme Eng. 5, 369–371.
Hilhorst, R., Spruijt, R., Laane, C., and Veeger, C.. (1984),Eur. J. Biochem. FEBS 144, 459–466.
Ruckenstein, E. and Karpe, P. (1990),Biotechnol. Lett. 12, 241–246.
Luthi, P. and Luisi, P. L. (1984),J. Am. Chem. Soc. 106, 7285–7286.
Serralheiro, M. L., Empis, J. M., and Cabrai, J. M. S. (1990),Biotechnol. Lett. 12, 167–172.
Skrika, A. E., Muir, J., and Freedman, R. B. (1993),Biotechnol. Bioeng. 41, 894–899.
Chang, G. and Shiao, S. (1994),Eur. J. Biochem. 220, 861–870.
Alexandrov, V. Ya. (1978),in Cells, Macromolecules and Temperature, Springer-Verlag, Berlin.
Miller, G. L. (1959),Anal. Chem. 31, 426–428.
Creighton, T. E. (1983), inProteins, Structure and Molecular Properties, Freeman, New York.
Klibanov, A. M. (1983),Adv. Appl. Microbiol. 29, 1–28.
Mao, Q., Xu, J., Ying, X., and Hu, Y. (1992),Huadong Huagong Xueyuan Xuebao,18, 276–280.
Combes, D. (1992),Prog. Biotechnol. 8, 45–52.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Subramani, S., Shah, C. & Madamwar, D. Stability of invertase in reverse micelles. Appl Biochem Biotechnol 60, 33–39 (1996). https://doi.org/10.1007/BF02788057
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02788057