Abstract
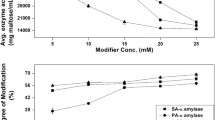
α-amylase (3.2.1.1) was solubilized in reverse micelles formed by Triton X-100 in xylene. Although the enzyme shows decrease in specific activity in reverse micellar medium, it possesses significantly high stability in comparison to bulk aqueous medium. Water/Surfactant ratio (Wo) was found to play a crucial role in both activity and stability of the enzyme. The optimum water/surfactant ratio for the catalytic function of an enzyme in reverse micelles is 36, while the enzyme is stable at Wo 12 for a considerably long period, and at Wo above 20 the enzyme gets inactivated within a day. Glycerol and CaCl2 improve the stability in both aqueous and reverse micellar medium. Thus the interior of the reverse micelles acts as a microreactor and provides favorable environment for the enzyme activity and stability.
Similar content being viewed by others
References
Arnold, F. H. (1990),TIBTECH 8 244–249.
Dordick, J. S. (1989),Enzyme Microb. Technol. 11 194–211.
Laane, C., Boren, S., Vos, K., and Veeger, C. (1987),Biotechnol. and Bioeng. 30 81–87.
Dickinson, M. and Fletcher, P. D. I. (1989),Enzyme Microb. Technol. 11 55–56.
Blanco, R. M. and Hailing, P. J. (1992),Biotechnol. and Bioeng. 39 75–84.
Inada, Y. and Matsushima, A. (1990),Biocatalysis 3 317–328.
Luisi, P. L., Giomini, M., Pileni, M. P., Robinson, B. H. (1988),Biochim. Biophys. Acta 947 209–246.
Schulman, J. H., Stoeckenous, W., and Prince, L. M. (1959),J. Phy. Chem. 63 1677–1680.
Danielsson, I. and Lindman, B. (1981),Colloids Surf. 3 391–392.
Leser, M. E., Wei, G., Luthi, P., Haering, G., Hochkoeppler, A., Blochliger, E., and Luisi, P. L. (1987),Journal de Chimie Physique 84 1113–1118.
Subramani, S. and Madamwar, D. (1995),Biotechnol. Tech. 9 45–48.
Ichikawa, S., Imai, M., and Shimizu, M. (1992),Biotechnol. and Bioeng. 39 20–26.
Wiseman, A. (1978), inTopics in Enzyme and Fermentation Biotechnology vol 2, Halsted & Sons, Chichester, 281–303.
Miller, G. L. (1959),Anal. Chem. 31 3, 426–428.
Lowry, O. H., Rosenberg, N. J., Farr, A. I., and Randall, R. J. (1951),J. Biol. Chem. 193 265–275.
Luisi, P., Meyer, P., and Wolf, R. (1980), inEnzyme Engineering 5 Plenum, New York, 369–371.
Pileni, M. P., Brochette, P., Hickel, B., and Larebours, B. (1984),J. Colloid Interface Sci. 98 549–558.
Barbaric, S. and Luisi, P. L. (1981),J. Am. Chem. Soc. 103 4239–4244.
Kabanov, A. V., Levashov, A. V. Klyachko, N. L., Namayotkin, S. N., Pshezhetsky, A. V., and Martinek, K. (1988),J. Theor. Biol. 133 327–343.
Adlercreutz, P. and Mattiason, B. (1987),Biocatalysis 1 99–108.
Serralheiro, M. L., Empis, J. M., and Cabral, J. M. S. (1990),Biotechnol. Lett. 12 (3) 167–172.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shah, C., Sellappan, S. & Madamwar, D. Role of environment on the activity and stability of α-amylase incorporated in reverse micelles. Appl Biochem Biotechnol 62, 183–189 (1997). https://doi.org/10.1007/BF02787994
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02787994