Abstract
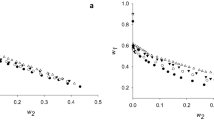
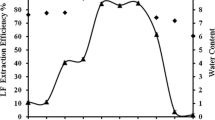
The recovery of α-amylase from the crude enzyme preparation by the reversed micellar liquid-liquid extraction was investigated. The reversed micellar solution was formed by dissolving a cationic surfactant Aliquat 336 in six different alkanes (cyclohexane, n-hexane, isooctane, n-octane, n-decane, and n-dodecane) respectively with addition of a cosolvent n-octanol. It was found that a minimal quantity of noctanol was needed for Aliquat 336 to dissolve in apolar solvent and form reversed micelles. Furthermore, this minimal amount of n-octanol needed was found to be different when Aliquat 336 was dissolved in different alkanes. It tended to increase with the number of carbon atoms in alkane and also depended on the solvent structure. During the forward extraction process, it was revealed that a high value of solubilization of protein in Aliquat 336 reversed micelles could be achieved when four out of the six alkanes (cyclohexane, n-hexane, isooctane, noctane) were used as the solvent for Aliquat 336. After a full forward and backward extraction cycle, however, a high recovery of both the protein mass and a-amylase activity in the stripping solution could be obtained only when two out of the six alkanes (n-hexane and isooctane) were used as the solvent for Aliquat 336. When n-hexane and isooctane were used as the solvent for Aliquat 336, up to 80% of the total α-amylase activity in the crude enzyme preparation could be recovered at the end of extraction cycle, meanwhile α-amylase could be concentrated about 1.4-fold. In the cases of other four alkanes (cyclohexane, n-octane, n-decane, and n-dodecane) as solvent, most of the α-amylase activity in the crude enzyme preparation would be denatured after an extraction cycle.
Similar content being viewed by others
References
Kadam, K. L. (1986), Reversed micelles as a bioseparation tool.Enzyme Microb. Technol. 8, 266–273.
Castro, M. J. M. and Cabral, J. M. S. (1988), Reversed micelles in biotechnology processes.Biotech. Adv. 6, 151–167.
Dekker, M., Hilhorst, R., and Laane, C. (1989), Isolating enzyme by reversed micelles.Anal. Biochem. 178, 217–226.
Luisi, P. L. and Laane, C. (1986), Solubilization of enzymes in apolar solvents via reversed micelles.Trends in Biotechnol. 4, 153–161.
Hatton, T. A. (1989), in: Scamehorn, J. F. and Harwell, J. H. eds.Surfactant—Based Separation. Marcel Dekker, New York. pp. 55–90.
Mat, H. B. and Stuckey, D. C. (1993), The effect of solvents on water solubilization and protein partitioning in reverse micellar systems, inSolvent Extraction in the Process Industries, vol.2. Logsdail, D. H. and Slater, M. J., eds. Published for SCI, Elsevier Applied Science, London and New York. pp. 933–938.
Dekker, M., Van’t Riet, K., Weijers, S. R., Baltussen, J. W. A., and Laane, C. (1986), Enzyme recovery by liquid-liquid extraction using reversed micelles.Chem. Eng. J. 33, B27–33.
Meier, P., Imre, E., Fleschar, M., and Luisi, P. L. (1984), Further investigations of the micellar solubilization of biopolymers in apolar solvents. inSurfactant in Solution. vol.2. Mittal, K. L. and Lindam, B. eds. Plenum, New York, pp. 999–1012.
Jolivalt, C., Minier, M., and Renon, H. (1990), Extraction of α-chymotrypsin using reversed micelles.J. Colloid Interf. Sci. 135, 85–96.
Lowry, O. H., Rosenbrough, N. J., Farr, A. L., and Randall, R. J. (1951), Protein mea-surement with the Folin Phenol reagent.J. Biol. Chem. 193, 265–275.
Bourrel, M. and Schechter, R. S. (1988),Microemulsions and Related System. Marcel Dekker, New York and Basel.
Marcozzi, G., Correa, N., Luisi, P. L., and Caselli, M. (1991), Protein extraction by reverse micelles: A study of the factors affecting the forward and backward transfer of α-chymotrypsin and its activity.Biotech. Bioeng. 38, 1239–1246.
Goklen, K. E. and Hatton, T. A. (1987), Liquid-liquid extraction of low molecular weight protein by selective solubilization in reversed micelles.Sep. Sci. Technol. 22, 831–841.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chang, QL., Chen, JY. Separation of α-amylase by reversed micellar extraction effect of solvent type and cosolvent concentration on the transfer process. Appl Biochem Biotechnol 62, 119–129 (1997). https://doi.org/10.1007/BF02787989
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02787989