Abstract
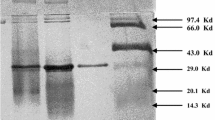
A thermostable cyclodextrinase (EC 3.2.1.54) fromBacillus stearothermophilus HY-1 was purified to homogeneity by disc-electrophoresis after sonication disruption, ammonium sulfate fractionation, DEAE-cellulose(DE32) column chromatography, hydroxyapatite chromatography, Sephadex G150 gel-filtration, and α-cyclodextrin-AH-Sepharose 4B affinity chromatography. The enzyme was purified 230-fold with 21.2% of activity recovery. The optimal substrates of the enzyme were α-, Β-, and γ-cyclodextrins and linear maltooligosaccharides, and the final product was mainly maltose. The enzyme could hydrolyze pullulan to produce panose. It could also hydrolyze soluble starch, amylose, and amylopectin, but not glycogen. The Km and Vmax for α-, Β-, and γ-cyclodextrins were 1.79, 1.67, and 2.50 mg/mL, and 336, 185, and 208 Μmol/mg/min, respectively. The molecular weight of the enzyme was 61,000 by SDS-gel-electrophoresis. The isoelectric point was pH 5.0. The enzyme was most active at pH 6.2 and 55‡C, and it was strongly inhibited by Cu2+, Hg2+, Zn2+, Pb2+, and slightly by Fe2+. The effect of some protein modification reagents on the activity of the enzyme suggested that tryptophan and histidine residue(s) may be located at the active site. The amino acid composition of the enzyme was also determined.
Similar content being viewed by others
Abbreviations
- CDase:
-
cyclodextrinase
- CDs:
-
cyclodextrins
- G1-G6:
-
glucose, maltose, maltotriose, maltotetraose, maltohexaose
- IAA:
-
iodo acetamide
- NEM:
-
N-ethylmaleimide
- NAI:
-
N-acetylimidazole
- DEPC:
-
diethylpyrocarbonate
- NBS:
-
N-bromosuccinimide
References
Tilden, E. B. and Hudson, C. S. (1942),J. Bacteriol. 43, 527–544.
Depinto, J. A. and Campbell, L. L. (1964),Science 146, 1064–1066.
Kitahata, S., Taniguchi, M., Betran, S. D., Suginodo, T., and Okada, S. (1983),Agric. Bid. Chem. 47, 1441–1447.
Oguma, T., Kikuchi, M., and Mizusawa, K. (1990),Biochim. Biophys. Acta 1036, 1–5.
Sah, B. C. and Zeikus, J. G. (1990),Appl. Environ. Microbiol. 56, 2941–2943.
Yoshida, A., Iwasaki, Y., Akiba, T., and Horikoshi, K. (1991),J. Fermit. Biozngin. 71, 226–229.
Kato, K., Sugimoto, T., Amemura, A., and Harada, T. (1975),Biochim. Biophys. Acta 391, 96–108.
Bender, H. (1981),Eur. J. Biochem. 115, 2879.
Enevoldsen, B. S., Reimann, L., and Hansen, N. L. (1977),FEBS Letters 79, 121–124.
Bernbeld, P. (1955),Methods in Enzymology 1, 149,150.
Lowry, O. H., Rosebrough, W. J., Farr, A. L., and Randall, R. J. (1951),J. Bid. Chem. 193, 265–275.
Davis, B. J. (1969),Ann. N.Y. Acad. Sci. 121, 404–427.
Weber, K. and Osborn, M. (1969),J. Bid. Chem. 244, 4406–4412.
Vesterberg, O. (1973),Science Tools 20, 22–29.
Kuriki, Y., Okada, S., and Imanaka, T. (1988),J. Bacteriol. 170, 1554–1559.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhong, W., Zheng, Z.S. & Jun, Y.S. Purification and properties of cyclodextrinase fromBacillus stearothermophilus HY-1. Appl Biochem Biotechnol 59, 63–75 (1996). https://doi.org/10.1007/BF02787858
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02787858