Abstract
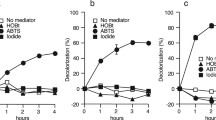
A fungal laccase (Myceliophthom thermophila) has been shown to function as an iodide oxidase. Unlike other halides which interact with the type 2 copper site and are inhibitors for the laccase, iodide interacts with the type 1 copper site and serves as a substrate capable of donating an electron to the laccase. Under anaerobic conditions, the interaction between the laccase and iodide results in the reduction of the laccase type 1 copper and the concomitant oxidation of iodide to form iodide. In aerated solutions, the laccase catalyzes the oxidation of iodide to iodine and the concomitant reduction of dioxygen to water. The reaction exhibits typical Michaelis kinetics with aK m of 0.16 ± 0.02M and ak cat of 2.7 ± 0.2 turnovers per min at the optimal pH (3.4). The catalysis can be enhanced by 2,2′-azino-bis-(3-ethylbenz-thiazoline-6-sulfonic acid), which shuttles electrons rapidly between iodide and the laccase. Bilirubin oxidase also demonstrates significant iodide oxidase activity, suggesting that the property could be a common feature for copper-containing oxidases. Possible industrial and medicinal applications for a laccase-based iodine production system are discussed.
Similar content being viewed by others
References
Reinhammar, B. and Malmstrom, B. G. (1981), inMetal Ions in Biology: Copper Proteins, vol. 3, Spiro, T. G., ed., Wiley, New York, pp. 109–149.
Farver, O. and Pecht, I. (1984), inCopper Proteins and Copper Enzymes, vol. 1, Lontie, R., ed., CRC Press, Boca Raton, pp. 183–214.
Mayer, A. and Harel, E. (1978),Phytochem. 18, 193–215.
Mayer, A. (1987),Phytochem. 26, 11–20.
Yaropolov, A. I., Skorobogat’ko, O. V., Vartanov, S. S., and Varfolomeyev, S. D. (1994),Appl. Biochem. Biotech. 49, 257–280.
Curzon, G. and Speyer, B. E. (1967),Biochem. J. 105, 243–250.
Koudelka, G. B. and Ettinger, M. J. (1988),J. Biol. Chem. 263, 3698–3705.
Winkler, M. E., Spira, D. J., LuBien, C. D., Thamann, T. J., and Solomon, E. I. (1982),Biochem. Biophys. Res. Comm. 107, 727–734.
Naki, A. and Varfolomeev, S. D. (1981),Biokhimiya 46, 1694–1702.
Murao, S. and Tanaka, N. (1981),Agric. Biol. Chem. 45, 2383–2384.
Hiromi, K., Tamaguchi, Y., Sugiura, Y., Iwamoto, H., and Hirose, J. (1992),Biosci. Biotech. Biochem. 56, 1349–1350.
Xu, F. (1995),FASEB J. 9, Abs498.
Berka, B. M., Thompson, S. A., Brown, S. H., Golightly, E. J., Brown, K. M., and Xu, F. (1994), abstr. Western Regional American Chemical Society Meeting and Pacific Conference, October 19–22, Sacramento, CA. A full report is in preparation.
Ramette, R. W. and Sandford, R. W. (1965),J. Am. Chem. Soc. 87, 5001–5005.
Childs, R. E. and Bardsley, W. G. (1975),Biochem. J. 145, 93–103.
Xu, F. Unpublished data.
Vanysek, P. (1993), inHandbook of Chemistry and Physics, Lide, D. R., ed., CRC Press, Boca Raton, pp. 8–19.
Bard, A. J., Parsons, R., and Jordan, J. (1985),Standard Potentials in Aqueous Solution, Marcel Dekker, New York, p. 292.
Sanchez-Ferrer, A., Rodriguez-Lopoz, J. N., Garcia-Canovas, F., and Garcia-Carmona, F. (1995),Biochim. Biophys. Acta 1247, 1–11.
Thomas, J. A. and Hager, L. P. (1969),Biochem. Biophys. Res. Comm. 35, 444–450.
Neidleman, S. L. and Geigert, J. (1981), U.S. Patent4,282,324.
Kessler, J. H. (1991), U.S. Patent 4,996,146.
Xu, F., Shin, W., Brown, S. H., Wahleithner, J.A., Sundaram, U., and Solomon, E. I. (1996),Biochim. Biophys. Acta 1292, 303–311.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xu, F. Catalysis of novel enzymatic iodide oxidation by fungal laccase. Appl Biochem Biotechnol 59, 221–230 (1996). https://doi.org/10.1007/BF02783566
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02783566