Abstract
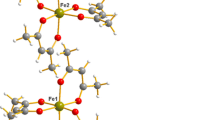
The structure of K4MnMo4O15 was solved from single-crystal X-ray diffraction data (a = 10372, c = 8.160 Å, Z = 2, space group P-3, 2152 reflections, R = 0.039). The structure is of new, glaserite-like, type. A characteristic and original feature of the structure is a Mn(II)O6 octahedron with six MoO4 tetrahedra attached to it by their vertices; the octahedron is linked with a MoO6 octahedron by a common face. The MoO4 tetrahedra bridge the octahedral dimers with each other, forming lacy layers with potassium atoms lying between the layers. K4M2+Mo4O15 (M2+= Mg, Co, Cd) phases, which have similar structures, have been synthesized and characterized.
Similar content being viewed by others
References
S. F. Solodovnikov, Z. A. Solodovnikova, P. V. Klevtsov, and E. S. Zolotova,Zh. Neorg. Khim.,39, No. 12, 1942–1947 (1994).
G. M. Sheldrick,SHELXL-93. Program for the Refinement of Crystal Structures, Göttingen (1993).
F. Scordari and G. Milella,Acta Crystallogr.,C39, No. 11, 1491–1493 (1983).
T. Araki,Z. Kristallogr.,194, Nos. 3–4, 161–191 (1991).
I. D. Brown and D. Altermatt,Acta Crystallogr.,B41, No. 4, 244–247 (1985).
V. K. Trunov, V. A. Efremov, and Yu. A. Velikodnyi,Crystal Chemistry and Properties of Binary Molybdates and Tungstates [in Russian], Nauka, Leningrad (1986).
N. V. Belov, L. P. Otroshchenko, and V. I. Simonov,Kristallografiya,29, No. 1, 44–49 (1984).
K. Okada and J. Ossaka,Acta Crystallogr.,B36, No. 4, 919–921 (1980).
P. B. Moore,Bull. Miner.,104, 536–547 (1981).
M. Seieborg,Acta Chem. Scand.,21, No. 2, 499–504 (1967).
S. A. Magarill and R. F. Hevtsova,Kristallografiya,16, No. 4, 742–745 (1971).
G. D. Fallon and B. M. Gatehouse,J. Solid State Chem.,44, No. 2, 156–161 (1982).
M. Touboul, C. Idoura, and P. Toledano,Acta Crystallogr.,C40, No. 10, 1652–1655 (1984).
G. Coquerel, C. Gicquel-Mayer, M. Mayer, and G. Perez,Acta Crystallogr.,C39, No. 12, 1602–1604 (1983).
N. M. Kozhevnikova, and M. V. Mokhosoev,Zh. Neorg. Khim.,37, No. 11, 2395–2401 (1992).
Author information
Authors and Affiliations
Additional information
Translated from Zhumal Strukturnoi Khimii, Vol. 38, No. 1, pp. 104–110, January–February, 1997.
Rights and permissions
About this article
Cite this article
Solodovnikov, S.F., Zolotova, E.S. & Solodovnikova, Z.A. Crystal structure of K4MnMo4O15 — A parent compound of a new isostructural series of complex oxides K4M2+Mo4O15 (M2+ = Mn, Mg, Co, Cd). J Struct Chem 38, 83–88 (1997). https://doi.org/10.1007/BF02768811
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02768811