Abstract
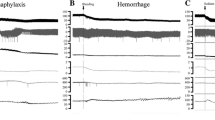
The role of endogenous vasopressin was studied in the development of mucosal erosions induced by haemorrhagic shock in the duodenum of the rat. Ischaemia-reperfusion provoked duodenal haemorrhagic lesions and elevated circulating and intramucosal vasopressin level. This mucosal injury was significantly attenuated by a vasopressin pressor receptor antagonist. Moreover, in the vasopressin-deficient Brattleboro homozygous rat, mucosal injury induced by haemorrhagic shock was also reduced. By contrast, when the vasopressin agonist, lysin-vasopressin, was administered, significant aggravation of ischaemia-reperfusion-induced duodenal mucosal injury was seen. These findings indicate the aggressive role of endogenous vasopressin, via its pressor receptors, in the generation of duodenal mucosal stress erosions in haemorrhagic shock.
Similar content being viewed by others
References
Robert A, Kauffman GL. Stress ulcers, erosions, and gastric mucosal injury. In: Sleisenger MH, Fordtran JS, eds. Gastrointestinal Disease. Pathophysiology, Diagnosis, Management. Philadelphia: Saunders; 1989:772–92.
Ben-Manachem T, Fogel R, Patel RV. Profilaxis for stress-related gastric hemorrhage in the medical intensive care unit. Ann Intern Med. 1994;121:568–75.
St-Louis J, Schiffrin EL. Biological actions and binding sites for vasopressin on the mesenteric artery from normal and sodium depleted rats. Life Sci. 1984;35:1489–95.
Laszio F, Karacsony G, Pavo I, Varga CS, Rojik I, Laszio FA. Aggressive role of vasopressin in development of different gastric lesions in rats. Eur J Pharmacol. 1994;258:15–22.
Hourani SMO, Cusack NJ. Pharmacological receptors on blood platelets. Pharmacol Rev. 1991;43:243–98.
Siess W, Stiefel M, Binder H, Weber PC. Activation of V1-receptors by vasopressin stimulated inositol phospholipid hydrolysis and arachidonate metabolism in human platelets. Biochem J. 1986;233:83–91.
Michell RH, Kirk CJ, Billah MM. Hormonal stimulation of phosphatidyl-inositol breakdown, with particular reference to the hepatic effect of vasopressin. Biochem Soc Trans. 1979;7:861–5.
Laszlo FA, Laszlo F, de Wied D. Pharmacology and clinical perspectives of vasopressin antagonists. Pharmacol Rev. 1991;43:73–108.
Manning M, Sawyer WH. Synthesis and receptor specificities of vasopressin antagonists. J Cardiovasc Pharmacol. 1986; Suppl 7:S29–35.
Laszlo F, Whittle BJR. Constitutive nitric oxide modulates the injurious actions of vasopression on rat intestinal microcirculation in acute endotoxaemia. Eur J Pharmacol. 1994;260:265–8.
Sokol HW, Valtin H, eds. The Brattleboro rat. Ann NY Acad Sci. 1982;394:1–828.
Leung FW, Itoh H, Hirabayashi K, Guth PH. Role of blood flow in gastric and duodenal mucosal injury in the rat. Gastroenterology. 1985;88:281–9.
Reichlin S. Neuroendocrinology. In: William RH, ed., Textbook of Endocrinology. Philadelphia: Saunders; 1985:492–511.
Melville RJ, Forsling ML, Frizis HI, LeQuesne LP. Stimulus of vasopressin release during elective intra-abdominal operations. Br J Surg. 1985;72:929–35.
Robertson GL. Posterior pituitary. In Baxter JD, ed., Endocrinology and Metabolism. New York: McGraw-Hill; 1987:335–56.
Share L. Role of vasopressin in cardiovascular regulation. Physiol. Rev. 1988;68:1248–84.
Liard JF, Deriaz P, Schelling P, Thibonnier M. Cardiac output distribution during vasopressin infusion or dehydration in conscious dogs. Am J Physiol. 1982;243:H663–9.
Baker CH, Sutton ET, Zhou Z, Deitz JR. Microvascular vasopressin effects during endotoxin shock in the rat. Circ Shock. 1990;30:81–95.
Vanner S, Jiang M-M, Brooks VL, Surprenant A. Characterization of vasopressin actions in isolat submucosal arterioles of the intestinal microcirculation. Circ Res. 1990;67:1017–26.
McNeill JR, Stark RD, Greenway CV. Intestinal vasoconstriction after hemorrhage: role of vasopressin and angiotensin. Am J Physiol. 1970;219:1342–7.
Stark RD, McNeill JR, Greenway CV. Sympathetic and hypophyseal roles in the splenic response to hemorrhage. Am J Physiol 1970;22:837–40.
Filep J, Rosenkrantz B. Mechanism of vasopressin-induced platelet aggregation. Thromb Res. 1987;45:7–16.
Nadasy GL, Szekacs B, Juhasz I, Monos E. Pharmacological modulation of prostacyclin and thromboxane production of rat and cat venous tissue slices. Prostaglandins. 1992;44:339–55.
Rosenbar GA, Kyner WT, Fenstermacher JD, Patlak CS. Effect of vasopressin on ependymal and capillary permeability to tritiated water in cat. Am J Physiol. 1986;251:F485–9.
Doczi T, Szerdahelyi P, Gulya K, Kiss J. Brain water accumulation after the central administration of vasopressin. Neurosurgery. 1982;11:402–6.
Laszlo F, Karacsony G, Szabo E, Lang J, Balaspiri L, Laszlo FA. The role of vasopressin in the pathogenesis of ethanol-induced gastric hemorrhagic erosions in rats. Gastroenterology. 1991;101: 1242–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
László, F., Szepes, Z., Karácsony, G. et al. Endogenous vasopressin damages duodenal mucosa during haemorrhagic shock in rats. Inflammopharmacology 4, 379–385 (1996). https://doi.org/10.1007/BF02755790
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02755790