Abstract
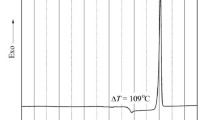
Lithium borosulphate glasses have been prepared in three different series: (a) (42·5 −x)Li2O:57·5 B2O3:xLi2SO4; (b) 42·5Li2O: (57·5 −x)B2O3:xLi2SO4 and (c) 42·5Li2O:57·5B2O3:xLi2SO4. The glass transition temperature (T g) of these glasses has been analysed on the basis of the fraction of four coordinated boron which governs the glass structure. The analysis reveals that the addition of Li2SO4 in series (a) and (b) gives rise to increased value of N4 whereas, in series (c) it increases the number of non-bridging oxygens.
Similar content being viewed by others
References
Bray P J and O’Keefe J G 1963Phys. Chem. Glasses 4 37
Button D P, Tandon R P, King C, Velez M H, Tuller H L and Uhlmann D R 1982J. Non-Cryst. Solids 49 129
Button D P, Moon P K, Tuller H L and Uhlmann D R 1983Glastech. Ber. 56 K Bd 2 856
Charles R J 1966J. Am. Ceram. Soc. 49 55
Deshpande V K, Rokade S and Singh K 1985Proc. Sixth Riso. Int. Symp. on transport-structure relationship in fast ion and mixed conductors, Denmark (eds) F W Poulsen, N H Andersen, K Clusen, S Skaarup and O T Sorensen; GH-Tryk 1/S Odense, 129
Gandhi P R, Deshpande V K and Singh K 1989Solid State Ionics 36 97
Ito Y, Miyauchi K and Oi T 1983J. Non-Cryst. Solids 57 389
Konijnedijk W N and Stevels J M 1975J. Non-Cryst. Solids 18 307
Kamitsos E I, Kavakassites M A and Chryssikos G D 1986J. Phys. Chem. 90 4528
Kamitsos E I, Kavakassites M A and Chryssikos G D 1987J. Phys. Chem. 91 5807
Levasseur A, Kabala M, Brethous J C and Hagenmullar P 1979aSolid State Commun. 32 839
Levasseur A, Brethous J C, Reau J M and Hagenmullar P 1979bMater. Res. Bull. 14 921
Otto K 1966Phys. Chem. Glasses 7 29
Singh K and Rokade S 1984J. Power Sources 13 159
Soppe W, Marcel C V D and Hartog H W D 1988J. Non-Cryst. Solids 101 101
Takahashi T and Yamamoto O 1979Chem. Lett. 135
Tuller H L and Button D P 1985Proc. Sixth Riso. Int. Symp. on Transport-structure relationship in fast ion and mixed conductors, Denmark (eds) F W Poulsen, N H Andersen, K Clusen, S Skaarup and O T Sorensen; GH-Tryk 1/S Odense, 119
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gandhi, P.R., Deshpande, V.K. & Singh, K. Lithium borosulphate glasses: an analysis of glass transition temperature results. Bull. Mater. Sci. 15, 467–471 (1992). https://doi.org/10.1007/BF02745297
Issue Date:
DOI: https://doi.org/10.1007/BF02745297