Abstract
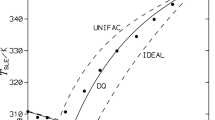
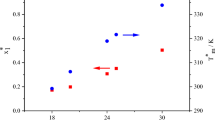
The phase diagram of a binary organic system involving diphenyl and succinonitrile shows the formation of a eutectic (0·968 mole fraction of succinonitrile) and a monotectic (0·074 mole fraction of succinonitrile) with a large miscibility gap in the system, the upper consolute temperature being 53·5°C above the monotectic horizontal. From the enthalpy of fusion of the pure components, the eutectic and the monotectic, determined by the DSC method, the enthalpy of mixing, Jackson’s roughness parameter, interfacial energy, size of the critical nucleus and excess thermodynamic functions were calculated.
Similar content being viewed by others
References
Christian J W 1965The theory of phase transformations in metals and alloys (Oxford: Pergamon Press)
Ecker A, Frazier D O and Alexander J I D 1989Metall. Trans. A20 2517
Elliott R 1977Int. Met. Rev. 22 161
Elliott R 1983Eutectic solidifcation processing (London: Butterworths)
Favite G 1996J. Cryst. Growth 166 29
Glazer J 1995Int. Mater. Rev. 40 65
Glicksman M E, Singh N B and Chopra M 1983Manufacturing in Space 11 207
Grugel R N and Poorman R 1989Mater. Sci. Forum 50 89
Herlach D M, Cochrane R F, Egry I, Fecht H J and Greer A L 1993Int. Mater. Rev. 38 273
Rai U S and Mandal K D 1990Mol. Cryst. Liq. Cryst. 182 387
Rai U S and Shekhar H 1994Cryst. Res. Technol. 29 551
Rai U S and George S 1996J. Thermal Anal. 46 1809
Rai U S and Rai R N 1996Thermochim. Acta 277 209
Rai U S, Singh O P, Singh N P and Singh N B 1983Thermochim. Acta 71 373
Rai U S, Singh O P and Singh N B 1987Can. J. Chem. 65 2639
Rzyman K, Moser Z, Watson R E and Weinert M 1996J. Phase Equilibria 17 173
Sangster J 1994J. Phys. Chem. Ref. Data 23 295
Singh N B 1978Acta Ciencia Indica 4 4
Singh N, Singh N B, Rai U S and Singh O P 1985Thermochim. Acta 95 291
Trivedi R and Kurz W 1994Int. Mater. Rev. 39 49
Wisniak J and Tamir A 1978Mixing and excess thermodynamic properties (A literature source book), inPhys. Sci. Data (New York: Elsevier)
Yasuda H, Ohnaka I, Matsunaga Y and Shiohara Y 1996J. Cryst. Growth 156 128
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rai, U.S., Rai, R.N. Phase diagram and thermochemical properties of organic eutectic in a monotectic system. Bull Mater Sci 21, 203–206 (1998). https://doi.org/10.1007/BF02744970
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02744970