Abstract
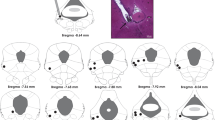
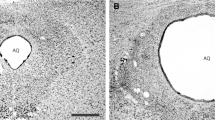
Defensive responses to a cat were observed in rats given excitotoxic lesions of the central nucleus of the amygdala (ACe), dorsolateral periaqueductal gray (dlPAG), ventral periaqueductal gray (vPAG), or sham lesions. Rats were placed adjacent to a compartment containing a cat. Sham-lesioned rats avoided the area nearest the cat and preferred the area furthest away from the cat. They also exhibited numerous defensive responses including, climbing, escape from the apparatus, and freezing. Rats with lesions of the ACe reacted like the sham lesioned rats by preferring the area of the apparatus furthest from the cat, however they climbed and escaped significantly less than sham lesioned rats. Avoidance of the area adjacent to the cat was attenuated in rats with lesions of the vPAG. Climbing along the walls of the apparatus was also attenuated in rats with lesions of the vPAG. Escapes from the apparatus were not significantly reduced by lesions of the vPAG and dlPAG. Thus, ACe lesions attenuated climbing and eliminated escapes, but did not impair locomotion of the rat away from the cat.
Similar content being viewed by others

References
Adamec, R. (2001). Does long term potentiation in periaqueductal gray (PAG) mediate lasting changes in rodent anxiety-like behavior (ALB) produced by predator stress? Effects of low frequency stimulation (LFS) of PAG on place preference and changes in ALB produced by predator stress.Behavioural Brain Research, 120(2), 111–135.
Alheid, G., Do Olmos, J.S., & Beltramino, C.A. (1995). Amygdala and extended amygdala. In Paxinos (Ed.)The rat nervous system (pp. 495–578). New York: Academic.
Amorapanth, P., LeDoux, J.E., & Nader, K. (2000). Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus.Nature Neuroscience, 3(1), 74–79.
Bandler, R. & Shipley, M.T. (1994). Columnar organization in the midbrain periaqueductal gray: modules for emotional expression?Trends in Neurosciences, 17(9), 379–389.
Bellgowan, P.S.F., & Helmstetter, F.J. (1996). Neural systems for the expression of hypoalgesia during nonassociative fear.Behavioral Neuroscience, 110(4), 727–736.
Blanchard, D.C. (1997). Stimulus, environmental, and pharmacological control of defensive behaviors. In M.E. Bouton & M.S. Fanselow (Eds.)Learning, Motivation and Cognition. Washington D.C.: American Psychological Association.
Blanchard, D.C. & Blanchard, R.J. (1972). Innate and conditioned reactions to threat in rats with amygdaloid lesions.Journal of Comparative and Physiological Psychology, 81(2), 281–290.
Blanchard, D.C. & Takahashi, S.N. (1988). No change in intermale aggression after amygdala lesions which reduce freezing.Physiology & Behavior, 42(6), 613–616.
Blanchard, R.J., & Blanchard, D.C. (1971). Defensive reactions in the albino rat.Learning and Motivation, 2, 351–362.
Blanchard, R.J., & Blanchard, D.C. (1989). Antipredator defensive behaviors in a visible burrow system.Journal of Comparative Psychology, 103, 70–82.
Blanchard, R.J., Flannelly, K.J., & Blanchard, D.C. (1986). Defensive behaviors of laboratory and wildRattus norvegicus.Journal of Comparative Psychology, 100(2), 101–107.
Bolles, R.C. (1971). Species-specific defense reactions. In F.R. Brush (Ed.),Aversive conditioning and learning. New York: Academic Press.
Bouton, M.E., & Bolles, R.C. (1980). Conditioned fear assessed by freezing and by the suppression of three different baselines.Animal Learning & Behavior, 8, 429–434.
Campeau, S., & Davis, M. (1995). Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimulus.Journal of Neuroscience, 15, 2301–2311.
Choi, J.S., & Brown, T.H. (2003). Central amygdala lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses.Journal of Neuroscience, 23(25), 8713–8721.
Coss, R.G. & Owings, D.H. (1978). Snake-directed behavior by snake naive and experienced California ground squirrels in a simulated burrow.Zeitschrift fuer Tierpsychologie, 48(4), 421–435.
de Oca, B.M., DeCola, J.P., Maren, M.S., & Fanselow, M.S. (1998). Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses.Journal of Neuroscience, 18(9), 3426–3432.
Diamond, D.M., & Rose, G.M. (1994). Stress impairs LTP and hippocampal-dependent memory.Annals of the New York Academy of Sciences, 746, 411–414.
Fanselow, M.S. (1991). The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In A. Depaulis & R. Bandler, (Eds.)The midbrain periaqueductal gray matter: Functional, anatomical, and neurochemical organization. Plenum Press: New York.
Fanselow, M.S. (1994). Neural organization of the defensive behavior system responsible for fear.Psychonomic Bulletin and Review, 1(4), 429–438.
Fanselow, M.S. (1997). Species-specific defense reactions: Retrospect and Prospect. InLearning, Motivation, and Cognition. M.E. Bouton, & M.S. Fanselow (Eds.). Washington D.C.: American Psychological Association.
Fanselow, M.S. & Lester, L.S. (1988). A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In R.C. Bolles, & M.D. Beecher (Eds.).Evolution and learning. Hillsdale, NJ: Lawrence Erlbaum Associates.
Fanselow, M.S., Lester, L.S., & Helmstetter, F.J. (1988). Changes in feeding and foraging patterns as an antipredator defensive strategy: A laboratory simulation using aversive stimulation in a closed economy.Journal of the Experimental Analysis of Behavior, 50, 361–374.
Fanselow, M.S., Sigmundi, R.A. & Williams, J. (1987). Response selection and the hierarchical organization of species-specific defense reactions: The relationship between freezing, flight, and defensive burying.Psychological Record, 37, 381–386.
Fox, R.J. & Sorensen, C.A. (1994). Bilateral lesions of the amygdala attenuate analgesia induced by diverse environmental challenges.Brain Research, 648(2), 215–221.
Griffith, C.R. (1920). The behavior of white rats in the presence of cats.Psychobiology, 2, 19–28.
Hebert, M.A., Ardid, D., Henrie, J.A., Tamashiro, K., Blanchard, D.C., & Blanchard, R.J. (1999). Amygdala lesions produce analgesia in a novel, ethologically relevant acute pain test.Physiology and Behavior, 67(1), 99–105.
Helmstetter, F.J. (1992). The amygdala is essential for the expression of conditional hypoalgesia.Behavioral Neuroscience, 106, 518–528.
Helmstetter, F.J. & Fanseow, M.S. (1993). Aversively motivated changes in meal patterns of rats in a closed economy: The effects of shock density.animal Learning & Behavior, 21, 168–175.
Kalin, N.H., Shelton, S.E., Davidson, R.J., & Kelley, A.E. (2001). The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament.The Journal of Neuroscience, 21(6), 2067–2074.
Killcross, S, Robbins, T.W., & Everitt, B.J. (1997). Different types of fear-conditioned behavior mediated by separate nuclei within amygdala.Nature, 388(24), 377–380.
Kim, J.J., Lee, H.J., Han, J.S., & Packard, M.G. (2001). Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning.Journal of Neuroscience, 21(14), 5222–5228.
LeDoux, J.E. (1993). Emotional memory: in search of systems and synapses.Annals of the New York Academy of Sciences, 702, 149–157.
LeDoux, J.E., Iwata, J., Cicchetti, P., & Reis, D.J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear.Journal of Neuroscience, 8, 2517–2529.
Lester, L.S. & Fanselow, M.S. (1985). Exposure to a cat produces opioid analgesia in rats.Behavioral Neuroscience, 99, 756–759.
Li, C.I., Maglinao, T.L., & Takahashi, L.K. (2004). Medial amygdala modulation of predator odor-induced unconditioned fear in the rat.Behavioral Neuroscience, 118(2), 324–332.
Maren, S. (1998). Overtraining does not mitigate contextual fear conditioning produced by neurotoxic lesions of the basolateral amygdala.Journal of Neuroscience, 18(8), 3088–3097.
Maren, S., Aharonov, G., Stote D.L., & Fanselow, M.S. (1996). N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats.Behavioral Neuroscience, 110(6), 1365–1374.
McGregor, I.S. & Dielenberg, R.A. (1999). Differential anxiolytic efficacy of a benzodiazepine on first versus second exposure to a predatory odor in rats.Psychopharmacology, 147(2), 174–181.
Ramos, C., Leite-Panissi, A., & Menescal-de-Oliveira, L. (2002). Central nucleus of the amygdala and the control of tonic immobility in guinea pigs.Brain Research Bulletin, 58(1), 13–19.
Roesler, R., Vianna, M.R.M., de-Paris, F., Rodrigues, C., Sant’Anna, M.K., Quevedo, J., & Ferreira, M.B.C. (2000). NMDA receptor antagonism in the basolateral amygdala blocks enhancement of inhibitor avoidance learning in previously trained rats.Behavioural Brain Research, 112(1–2), 99–105.
Satinder, K.P. (1976). Reactions of selectively bred strains of rats to a cat.Animal Learning & Behavior, 4 (2), 172–176.
Selye, H. (1976).Stress in health and disease. Boston: Butterworths.
Silveira, M.C.L., Sandner, G., & Graeff, F.G. (1993). Induction of Fos immunoreactivity in the brain by exposure to the elevated plus maze.Behavioural Brain Research, 56, 115–118.
Ulrich, R.E. & Azrin, N.H. (1962). Reflexive fighting in response to aversive stimulation.Journal of the Experimental Analysis of Behavior, 5, 511–520.
Walker, D.L. & Davis, M. (1997). Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear.Journal of Neuroscience, 17(23), 9375–9383.
Walker, D.L., Toufexis, D.J., & Davis, M. (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety.European Journal of Pharmacology, 463, 199–216.
Wallace, K.J. & Rosen, J.B. (2000). Predator odor as an unconditioned fear stimulus in rats: Elicitation of freezing by trimethylthiazoline, a component of fox feces.Behavioral Neuroscience, 114(5), 912–922.
Wiedenmayer, C.P., Goodwin, G.A., & Barr, G.A. (2000). The effect of periaqueductal gray lesions on responses to age-specific threats in infant rats.Developmental Brain Research, 120, 191–198.
Zangrossi, H., Jr. & File, S.E. (1994). Habituation and generalization of phobic responses to cat odor.Brain Research Bulletin, 33, 189–194.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oca, B.M., Fanselow, M.S. Amygdala and periaqueductal gray lesions only partially attenuate unconditional defensive responses in rats exposed to a cat. Integr. psych. behav. 39, 318–333 (2004). https://doi.org/10.1007/BF02734170
Issue Date:
DOI: https://doi.org/10.1007/BF02734170